pH Mystery Lab

2
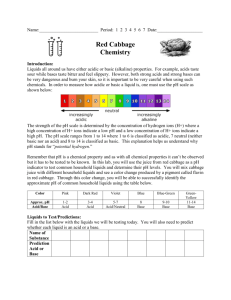
pH mystery lab
Intro: pH is the concentration of hydrogen ions in a solution. The higher the concentration of hydrogen ions the more acidic a solution is. Within the human body, we have a variety of liquids with varying pH from the blood with a slightly basic pH at 7.45 and stomach acid (hydrochloric acid) at 1. It is very important to understand pH for biology. pH can affect things such as chemical reactions and metabolism processes in our body. If we are not careful, we can mess up the pH balance in our bodies and things can go wrong.
Materials:
All students must have chemical goggles on at all times. The student that is performing the testing, must have gloves on at all times.
Directions:
There are 10 common household and biological liquids at the front desk. As scientists, it is your job to figure out what each item is using the dichotomous key provided. As you identify the solutions fill out the table below with the information it asks for. There will be rotating beakers of solution. When you get each beaker write down any observations about the liquid. DO
NOT TASTE ANY LIQUIDS IN THE LAB. Put a pH strip on top of a paper towel. Using the eyedropper provided in each liquid, put a single drop onto the pH paper. Match the color of the strip to the color guide on the side of the pH strip container. After all solutions have gone around identify the solutions using the dichotomous key.
# observation pH
What is it?
1
5
4
3
6
7
10
9
8
Lab Questions:
1. What was the most acidic liquid that was tested?
2. What was the most basic liquid tested?
3. Which result was most unexpected to your group and why? pH balance is very important to the human body. Use the following article to read about pH balance in the body to find why it’s important.
http://en.wikipedia.org/wiki/Acid%E2%80%93base_homeostasis
4. In the introduction, it talks about the effects of losing pH balance. What are possible outcomes of losing pH balance( there are 3 mentioned).
5. Under the section, Imbalance, what is it called when the blood it too acidic?
6. In the same section, what is it called when there’s too much base in the blood?
7. Under the section Mechanisms, what are 3 ways the body regulates pH?
8. Who were your group members?
pH Mystery Lab Dichotomous Key
1. Is it an acid?.................................................................................................................go to step 2
Is it a Base?.................................................................................................................Go to step 3
Is it Neutral?.........................................................................................................................Water
2. Is the liquid clear?......................................................................................................Go to step 4
Is the liquid opaque?....................................................................................................Go to step 5
3. pH of 10-12?.......................................................................................................................Bleach
pH of 7-8?..................................................................................................................Baking Soda
4. Liquid has no color?........................................................................................Hydrochloric Acid
Liquid has color?........................................................................................................Go to step 6
5. Liquid is white?......................................................................................................................Milk
Liquid is orange?........................................................................................................Go to step 7
6. pH of 1?.........................................................................................................Toilet Bowl Cleaner
pH above 1?................................................................................................................Go to step 8
7. pH of 2-3?..................................................................................................................Lemon Juice
pH of 4-5?.................................................................................................................Orange Juice
8. Liquid has bubbles if swirled?......................................................................................Coca-Cola
Liquid doesn’t have bubbles is swirled?...........................................................................Vinegar