High-pressure reduced-kinetics mechanism for n
advertisement

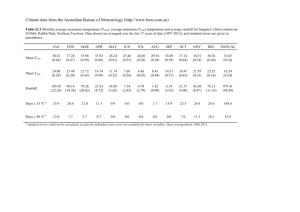
Combustion and Flame Supplementary material High pressure reduced-kinetics mechanism for n-hexadecane autoignition and oxidation at constant pressure by Panayotis D. Kourdisa and Josette Bellana,b* California Institute of Technology, Pasadena, CA 91125, USA Jet Propulsion Laboratory, California Institute of Technology, Pasadena, CA 91109, USA a) b) Figure S1. The tables S, Sn, Sn+ and Sn- are referred in Section 3.2. In matrices S n , S n and S n all columns except the first one, explicitly shown, are the same as in matrix S. * Corresponding author. Tel: (818) 354-6959, Fax: (818) 393-6682. Email address: Josette.Bellan@jpl.nasa.gov (Josette Bellan) 1/2 Combustion and Flame Supplementary material Figure S2. Left: Schematic overview of the parametrization of NCi as a function of Tn when T0=Tmin and Tfinal=Tmax. The resulting function NCi(Tn) is single-valued (SV) for all Tn [0,1]. Right: Schematic overview of the parametrization of NCi as a function of Tn when T0=Tmin and Tfinal<Tmax. The resulting function NCi(Tn) is single-valued (SV) for Tn [0,Tn,f[ and multi-valued (MV) for [Tn,f,1]. In both figures, the arrows indicate the direction of increasing time. 2/2