Translational Program Grant Progress Report
advertisement

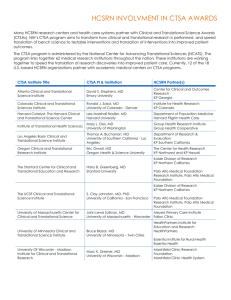
Translational Program Grant Progress Report Due: 31 March 2015 at 5pm Please complete this form in 10pt Verdana font Please note that any requests for variations to the grant should NOT be requested in this report. Any requests should be directed to the Grants Team via the Administering Institution. Refer to http://www.cancerinstitute.org.au/research-grants-and-funding/grants/policies-andforms for further details. Section A – Project Identification A.1 Project Information Grant ID No. Project Title Funding Commenced Funding End Date A.2 Chief Investigator Information Full Name Contact Number Email Address A.3 Administering Institution A.4 Research Institutions involved in this grant (insert additional rows as needed) Cancer Institute NSW – Translational Program Grant Progress Report E14/57744 Due date 5pm, 31/03/2015 Section B – Project Objectives and Progress B.1 Project Summary Please include here the original lay summary from your application (approx. 250 words): B.2 Research Progress B.2.1 Outline the progress achieved against your research plan for the reporting period: B.2.2 Have there been any changes to the project since your last report? ☐ Yes ☐ No If yes, please list and provide rationale: NOTE: Requests for variations to the budget, start or end date of the grant, Chief Investigators or Administering Institutions must not be requested within this report. Refer to http://www.cancerinstitute.org.au/research-grants-and-funding/grants/policies-and-forms for further details on requesting a variation to the grant. Cancer Institute NSW – Translational Program Grant Progress Report E14/57744 Due date 5pm, 31/03/2015 Section C – Research Achievements C.1 Summarise the program’s achievements in the T1, T2 and T3 (Please refer explicitly to achievements in the 3 stages of translation T1, T2 and T3, as defined in Appendix 1 and the guidance notes). Please include up to 3 achievements in each of the 3 stages of translational. Copy and paste the tables below as required. C.1.1 - T1 Description of Achievement (approx 500 words) Please choose one category that best describes this achievement: Increased the capacity to do further research Produced new knowledge Developed new diagnostic tools or new therapies Informed policy or practice Improved health outcomes Other - please describe: Who will this achievement most directly impact? Patients/Families Clinicians Public/Communities Other Researchers Other - please describe: C.1.2 - T2 Description of Achievement (approx 500 words) Cancer Institute NSW – Translational Program Grant Progress Report E14/57744 Due date 5pm, 31/03/2015 Please choose one category that best describes this achievement: Increased the capacity to do further research Produced new knowledge Developed new diagnostic tools or new therapies Informed policy or practice Improved health outcomes Other - please describe: Who will this achievement most directly impact? Patients/Families Clinicians Public/Communities Other Researchers Other - please describe: C.1.3 – T3 Description of Achievement (approx 500 words) Please choose one category that best describes this achievement: Increased the capacity to do further research Produced new knowledge Developed new diagnostic tools or new therapies Informed policy or practice Improved health outcomes Other - please describe: Who will this achievement most directly impact? Patients/Families Clinicians Public/Communities Other Researchers Other - please describe: Cancer Institute NSW – Translational Program Grant Progress Report E14/57744 Due date 5pm, 31/03/2015 C.2 How is the program delivering translational cancer research at the highest international standards? C.3 How is the program strengthening cancer research collaborations, networks and/or consortia to provide greater research depth? C.4 How is the program increasing research capacity and activity to address pressing clinical problems, in ways which would not have occurred otherwise? C.5 Is there any interest in commercialising your research? Cancer Institute NSW – Translational Program Grant Progress Report E14/57744 Due date 5pm, 31/03/2015 Section D - Research Outputs Please include references for all journal articles and conference presentations arising during the report period (only those directly relate to the funded program). Include the full reference (including authors and date of publication). (Insert additional rows as necessary). D.1.1 Journal Articles Type (Published or In Press/Accepted) Full Reference Year of Publication Translational Pipeline Category (T1/T2/T3) D.1.2 Research dissemination Type (Poster/ Oral/Keynote) Full Reference Location (Local/ National/ International) Year Translational Pipeline Category (T1/T2/T3) D.1.3 - Please include reference to any other outputs arising from the program (eg reports, patents etc) Type (Report/Book/ Patent/Other) Full Reference Cancer Institute NSW – Translational Program Grant Progress Report E14/57744 Year Due date 5pm, 31/03/2015 D.2 Clinical Trials Did the research contribute towards the development of any clinical trials? ☐ Yes ☐ No If yes, please list Trial Identifier (eg. ANZCTR no.) Year opened Trial Name D.3 Clinical Practice Guidelines Did the research contribute towards the development of any clinical practice guidelines? ☐ Yes ☐ No If yes, please list Area of clinical practice Cancer Institute NSW – Translational Program Grant Progress Report E14/57744 Full Reference Due date 5pm, 31/03/2015 E. Other Funding Please list any funding awarded in the report period where the Translational Program Grant assisted in some way in gaining the additional funding. Insert rows as needed: Please DO NOT duplicate funds reported from previous years Grant Type Grant Amount % of funds allocated to you Years Covered by Grant (e.g 20142016) Funding Source Cancer Institute NSW – Translational Program Grant Progress Report E14/57744 Grant Title Chief Investigators Extent to which CINSW grant assisted in gaining this funding (please rate from 0-10 10=CINSW grant essential, 0=would have occurred without CINSW grant) Due date 5pm, 31/03/2015 F. Workforce capacity building F.1. Please provide a summary of the FTE staff employed through this grant Researchers (include postdocs, research fellows, snr scientists) Clinicians/Clinical Fellows Research support staff (include RAs, project officers, Technical staff) Nurse/data managers Program/ Research Managers Other FTE employed through grant F.2. Training Opportunities developed Over the duration of this grant, please record if any higher degree research students have been supported by the funded personnel (through either supervision or use of supported infrastructure)? How many of these students completed their degree in this period? Type Number Number completed Honours Masters (Research) PhD Other (please describe) Cancer Institute NSW – Translational Program Grant Progress Report E14/57744 Due date 5pm, 31/03/2015 G. Certification G.1 Certification by Chief Investigator I certify that this is an accurate progress report for the period covered Name Date G.2. Certification by Head of Department/Unit I certify that this is an accurate progress report for the period covered Name Date G.3. Certification by Administering Institution I certify that this is an accurate progress report for the period covered Name Date Submission ● Please ensure that progress report has been certified by all parties. ● The Administering Institution is responsible for the accurate submission of completed progress report form. ● Please note signatures are not required. ● Please save your progress report using the following format for naming and in the email subject line: Grant ID #_2014ProgressReport_Surname (e.g. 02TPG108_2014ProgressReport_Smith). ● Email progress report to grants@cancerinstitute.org.au ● Please note, late submissions of progress reports may affect eligibility for future funding. Cancer Institute NSW – Translational Program Grant Progress Report E14/57744 Due date 5pm, 31/03/2015 APPENDIX 1: CANCER INSTITUTE NSW MODEL OF TRANSLATION Figure 1 below is the model of translational research used by the Cancer Institute NSW. This model focuses on the translation of basic research into clinical research as well as being applicable to the translation of population health and health services research informing programs and service delivery. Figure 1 outlines /classifies the stages of translational research as: T1- developing treatments and interventions. T2 - testing the efficacy and effectiveness of these treatments and interventions. T3 - dissemination and implementation research for system-wide change. Figure 1: Model of Translational Research Westfall et al., (2007). Practice-based research – “blue highways” on NIH roadmap. JAMA, 297(4): 403-406 (adaptation) NSW Health and Medical Research Strategic Review 2012—page 4 (adaption) Cancer Institute NSW – Translational Program Grant Progress Report E14/57744 Due date 5pm, 31/03/2015 Detailed Translational Pipeline Definitions: i. ii. iii. T1 - Translation to Humans Developing treatments and interventions The translation of basic research into research with humans. Basic research could cover a range of disciplines including: laboratory research, epidemiology, psychometrics, social science. The interface between basic research and the clinical setting, striving to find how new knowledge of disease mechanisms can be developed into clinically relevant understandings, and diagnostic and treatment regimes to be trialled in humans. Types of studies/activities – Observational studies, Case studies; Phase 1 and II clinical trials T2 - Translation to Patients Testing the efficacy and effectiveness of these treatments and interventions. The translation of new clinical science and knowledge into routine clinical practice and health decision making1. Translation of new clinically proven knowledge of disease processes, diagnostic or treatment techniques into routine clinical practice and health decision making. Types of studies/activities - Phase III clinical trials; observational studies; evidence synthesis and guidelines development. T3 - Translation to Practice Dissemination and implementation research for system-wide change2. The application of information and insights derived from basic, clinical and population health research to the provision of health services2. Moving evidence-based guidelines into health practice, through delivery, dissemination, and diffusion research. Practice based research, where the evidence from clinical trials on carefully selected patients is translated into guidelines for patients seen routinely in practice. Types of studies/activities - Dissemination research; implementation research; diffusion research, Phase IV clinical trials. 1 Grimshaw et al, 2012 2 Wills NSW Health and Medical Research Strategic Review Cancer Institute NSW – Translational Program Grant Progress Report E14/57744 Due date 5pm, 31/03/2015