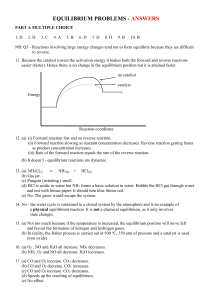
MYP 10 Equilibrium WS3
advertisement
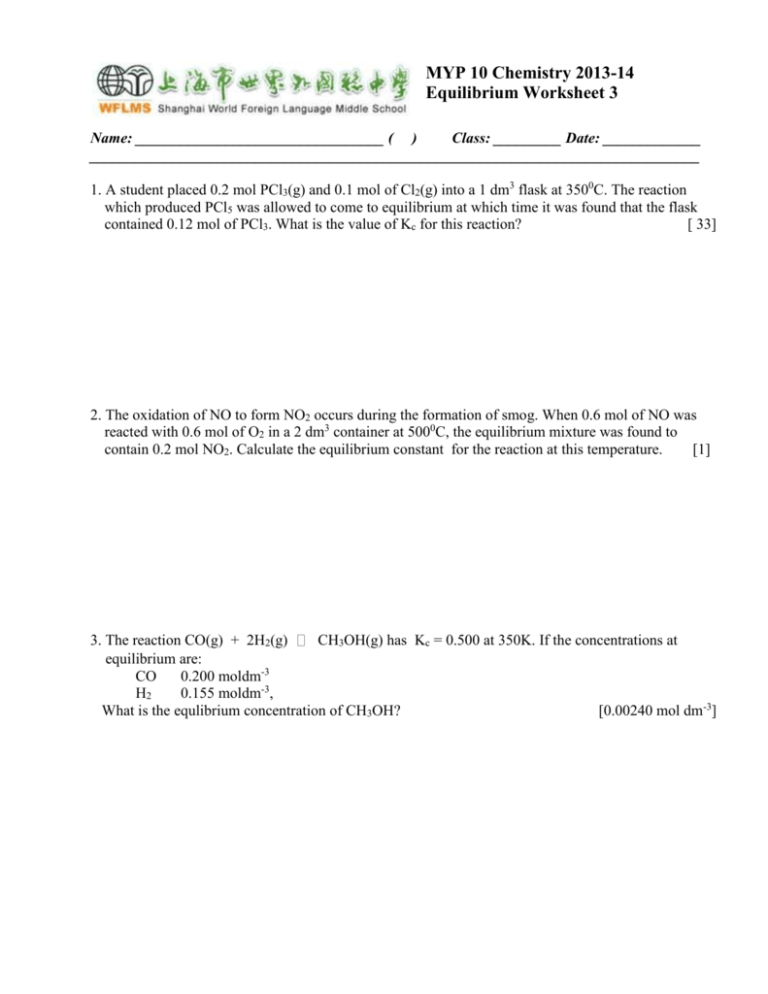
MYP 10 Chemistry 2013-14 Equilibrium Worksheet 3 Name: _________________________________ ( ) Class: _________ Date: _____________ _________________________________________________________________________________ 1. A student placed 0.2 mol PCl3(g) and 0.1 mol of Cl2(g) into a 1 dm3 flask at 3500C. The reaction which produced PCl5 was allowed to come to equilibrium at which time it was found that the flask contained 0.12 mol of PCl3. What is the value of Kc for this reaction? [ 33] 2. The oxidation of NO to form NO2 occurs during the formation of smog. When 0.6 mol of NO was reacted with 0.6 mol of O2 in a 2 dm3 container at 5000C, the equilibrium mixture was found to contain 0.2 mol NO2. Calculate the equilibrium constant for the reaction at this temperature. [1] 3. The reaction CO(g) + 2H2(g) CH3OH(g) has Kc = 0.500 at 350K. If the concentrations at equilibrium are: CO 0.200 moldm-3 H2 0.155 moldm-3, What is the equlibrium concentration of CH3OH? [0.00240 mol dm-3] 4. The equilibrium constant Kc for the reaction SO2(g) + NO(g) NO2(g) + SO2(g) was found to be 6.78 at a special temperature. If the initial concentrations of NO and SO3 were both 0.03 mol dm-3, what would be the equilibrium concentration of each component? 5. The thermal decomposition of water has a vey small value of Kc. At 10000C, Kc = 7.3 x 10-18 for the reaction. 2H2O(g) 2H2(g) + O2(g) A reaction is set up at this temperature with an initial H2O concentration of 0.10 mldm-3. Calculate the H2 concentration at equilibrium. [5.3 x 10-7 mol dm-3] Calculation of Qc 6. A mixture of 4.2 mol of N2, 2.0 mol of H2, and 10.0 mol of NH3 is introduced into a 20.0 L reaction vessel at 500K. At this temperature, the equiibrium constant Kc for the reaction N2(g) + 3H2(g) 2NH3(g) is 1.7 x 102. Is the reaction mixture at equilibrium If not, what is the direction of the reaction? [not at eqm, fr right to left, decreasing the conc of NH3 nd increasing the conc of N2 andH2 until Kc = 1.7 x 10 2] 7. The equilibrium constant Kc for the reaction 2 NO(g) + O2(g) 2NO2(g) is 6.9 x 105 at 500K. A 5.0L reaction vessel at this temperature was filled with 0.060 mol of NO, 1.0 mol of O2, and 0.80 mol of NO2. (a) Is the reaction mixture at equilibrium? If not, in which direction does the reaction proceed? (b) What is the direction of the reaction if the initial amounts are 5.0 x 10-3 mol of NO, 0.20 mol of O2, and 4.0 mol of NO2? 8. At 1400K, Kc = 2.5 x 10-3 for the reaction CH4(g) + 2H2S(g) CS2(g) + 4H2(g) . A 10.0 L reaction vessel at 1400K contains 2.0 mol of CH4, 3.0 mol of CS2, 3.0 mol of H2, and 4.0 mol of H2S. Is the reaction mixture at equilibrium? If not, in which direction does the recation proceed to reach equilibrium? [not at eqm, because Qc > Kc. The reaction will proceed from right to left to reach eqm] Others 1. At 800K, Kc= 4.24 for the reaction CO(g) + H2O(g) What is Kp at the same temperature? CO2(g) + H2(g). [4.24] 2. An equilibrium mixture of gaseous O2, NO, and NO2 at 500K contains 1.0 x 10-3M O2 and 5.0 x 10-2M NO2. At this temperature, the equiibrium constant Kc for the reaction 2NO(g) + O2(g) 2NO2(g) is 6.9 x 105. What is the concentration of NO? [1.9 x 10-3M] 3. The equiibrium constant Kc for the reaction of H2 and I2 is 57.0 at 700K. H2(g) + I2(g) 2HI(g) Kc = 57.0 at 700K If 1.00 mol H2 is allowed to react with 1.00 mol of I2 in a 10.0L reaction vessel at 700K, what are the concentrations of H2, I2 and HI at equilibrium? What is the composition of the equilibrium mixture in moles? [0.0209M,0.158M] 4. Calculate the equilibrium concentrations of H2, I2 and HI at 700K if the initial concentrations are [H2] = 0.100M and [I2] = 0.200M The equilibrium constant Kc for the reaction H2(g) + I2(g) 2HI(g) is 57.0 at 700K. [0.006M,0.106M,0.189M] 5. At certain temperature, the reaction PCl5(g) PCl3(g) + Cl2(g) has an equilibrium constant, Kc = -2 5.8 x 10 . Calculate the equilibrium concentrations of PCl5, PCl3 and Cl2 if only PCl5 is present initially, at a concentration of 0.160M. [(a)[PCl3]=[Cl2]=0.071M;[PCl5]=0.089M] 6. When each of the following equilibria is distribted by increasing the pressure as a result f decreasing the volume, does the number of oles of reaction products increase, decrease or remain the same? (a) 2CO2(g) 2CO(g) + O2(g) (b) N2(g) + O2(g) 2NO(g) (c) Si(s) + 2Cl2(g) SiCl4(g) [(a)dec (b) no ch (c) inc] 7. For the water-gas shift reaction CO(g) + H2O(l) CO2(g) + H2(g), ΔH= -41.2 kJ, does the amount of H2 in an equilibrium mixture increase or decrease when the temperature is increased? How does Kc change when the temperature is decreased?Justify your answers using Le Chatelier’s principle. [H2 dec when the temp is inc. As the temp is dec,the reaction shifts to the right. [CO2] and [H2] inc, [CO] and [H2O] dec, and Kc inc] 8. For the reaction H2(g) + I2(g) increase, decrease when: (a) I2 is added? (b) H2 is removed? (c) A catalyst is added? (d) The temperature is increased? 2HI(g) , ΔH= -9.4 kJ, will equilibrium concentration of HI [(a)inc(b)dec (c) no ch (d) dec] 9. Methanol (CH3OH) s manufactured by reaction of carbon mnoxide with hydrogen in the presence of a ZnO/Cr2O3 catalyst: ZnO/Cr2O3 CO(g) + 2H2(g) CH3OH(g) Does the amount of methanol increase, decrease or remain the same when an equilibrium mixture of reactants and products is subjected to the following: (a) The temperature is increased. (b) The volume is decreased. (c) Helium is added. (d) CO is added. (e) The catalyst is removed. [(a)dec(b)inc (c) no ch (d) inc (e)no ch]