Final_Biochemistry_Review
advertisement

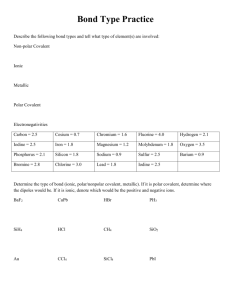
Biochemistry Quiz Review: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. What is the atomic number for Calcium? How many protons does nitrogen have? What does the prefix mono mean? Compare and contrast cohesion and adhesion. The space outside of the nucleus of an atom contains what? Compare and contrast acids and bases. Compare and contrast these three types of bonding: covalent, ionic, and hydrogen/polar. What is an ion? Solutions and suspensions are both mixtures. How are they different? Describe what makes a water molecule polar. What is a buffer, and why is it important? Biochemistry Quiz Review: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. What is the atomic number for Calcium? How many protons does nitrogen have? What does the prefix mono mean? Compare and contrast cohesion and adhesion. The space outside of the nucleus of an atom contains what? Compare and contrast acids and bases. Compare and contrast these three types of bonding: covalent, ionic, and hydrogen/polar. What is an ion? Solutions and suspensions are both mixtures. How are they different? Describe what makes a water molecule polar. What is a buffer, and why is it important? Biochemistry Quiz Review: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. What is the atomic number for Calcium? How many protons does nitrogen have? What does the prefix mono mean? Compare and contrast cohesion and adhesion. The space outside of the nucleus of an atom contains what? Compare and contrast acids and bases. Compare and contrast these three types of bonding: covalent, ionic, and hydrogen/polar. What is an ion? Solutions and suspensions are both mixtures. How are they different? Describe what makes a water molecule polar. What is a buffer, and why is it important?