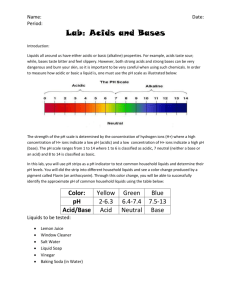
ANALYSIS QUESTIONS: These MUST be answered in complete
advertisement

ANALYSIS QUESTIONS: These MUST be answered in complete sentences that re-state the question. Also, you and your partner will collaborate on the answers, but please record your answers in alternating handwriting – you answer #1, your partner answers #2, etc. 1. Which substance tested from table A was the most acidic? Which substance was the most basic? 2. Did any substance from table A have a neutral, or near neutral pH? If so, which substance was neutral? 3. Our stomach contents are very acidic. Which ion is present in highest concentration in our stomachs? Which ion is present in highest concentration in antacids, such as Mylanta? 4. Describe the ion concentration of a neutral solution. What is the difference between the pH of 14 and a pH of 8? 5. Explain why the expected pH of water is 7. 6. Antacids dissolve readily in water. Using your knowledge of this chapter as well as good thinking skills, Describe the type of bonds that form an antacid compound. Hint: think of what is formed when the antacid dissolves or dissociates in water! ANALYSIS QUESTIONS: These MUST be answered in complete sentences that re-state the question. Also, you and your partner will collaborate on the answers, but please record your answers in alternating handwriting – you answer #1, your partner answers #2, etc. 1. Which substance tested from table A was the most acidic? Which substance was the most basic? 2. Did any substance from table A have a neutral, or near neutral pH? If so, which substance was neutral? 3. Our stomach contents are very acidic. Which ion is present in highest concentration in our stomachs? Which ion is present in highest concentration in antacids, such as Mylanta? 4. Describe the ion concentration of a neutral solution. What is the difference between the pH of 14 and a pH of 8? 5. Explain why the expected pH of water is 7. 6. Antacids dissolve readily in water. Using your knowledge of this chapter as well as good thinking skills, Describe the type of bonds that form an antacid compound. Hint: think of what is formed when the antacid dissolves or dissociates in water! ANALYSIS QUESTIONS: These MUST be answered in complete sentences that re-state the question. Also, you and your partner will collaborate on the answers, but please record your answers in alternating handwriting – you answer #1, your partner answers #2, etc. 1. Which substance tested from table A was the most acidic? Which substance was the most basic? 2. Did any substance from table A have a neutral, or near neutral pH? If so, which substance was neutral? 3. Our stomach contents are very acidic. Which ion is present in highest concentration in our stomachs? Which ion is present in highest concentration in antacids, such as Mylanta? 4. Describe the ion concentration of a neutral solution. What is the difference between the pH of 14 and a pH of 8? 5. Explain why the expected pH of water is 7. 6. Antacids dissolve readily in water. Using your knowledge of this chapter as well as good thinking skills, Describe the type of bonds that form an antacid compound. Hint: think of what is formed when the antacid dissolves or dissociates in water! ANALYSIS QUESTIONS: These MUST be answered in complete sentences that re-state the question. Also, you and your partner will collaborate on the answers, but please record your answers in alternating handwriting – you answer #1, your partner answers #2, etc. 1. Which substance tested from table A was the most acidic? Which substance was the most basic? 2. Did any substance from table A have a neutral, or near neutral pH? If so, which substance was neutral? 3. Our stomach contents are very acidic. Which ion is present in highest concentration in our stomachs? Which ion is present in highest concentration in antacids, such as Mylanta? 4. Describe the ion concentration of a neutral solution. What is the difference between the pH of 14 and a pH of 8? 5. Explain why the expected pH of water is 7. 6. Antacids dissolve readily in water. Using your knowledge of this chapter as well as good thinking skills, Describe the type of bonds that form an antacid compound. Hint: think of what is formed when the antacid dissolves or dissociates in water!