S2 acids and alkalis
advertisement

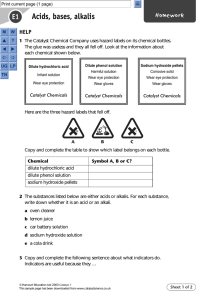
Learning Outcomes Having taken part in practical activities to compare the properties of acids and alkalis, I have demonstrated ways of measuring and adjusting pH and can describe the significance of pH in everyday life Concept Card If oranges contain citric acid, why don’t they poison you? There is a difference between acids in food and acids in the environment. They are poisonous, but the acid is not concentrated enough to be harmful. Not all acids are poisonous. Acids and Alkalis Acid, Alkali or Neutral Solutions Solutions can be classed as either acid, alkaline, or neutral. We use a scale called the pH scale in order to do this. The scale goes from pH 1 to 14. A liquid which changes colour called universal indicator can be added to solutions to match up to a number on the pH scale. This scale is shown here:- Acid, Alkali or Neutral Solutions Q1. Q2. Q3. Q4. Q5. What is the name of the scale used to classify solutions as acid, alkaline or neutral? Name the liquid added to solutions which changes colour to indicate if they are acid, alkali or neutral? Which numbers on the scale indicate a solution is an acid? Which numbers on the scale indicate a solution is an alkali? Which number on the scale indicates a solution is neutral? Collect a pH scale and stick it into your jotter. Experiment You are going to test some household substances to find out if they are acid, alkaline, or neutral. Collect a dimple tray and place a few drops of each substance on separate dimples. Add a drop of universal indicator to the substance and compare the colour with the pH chart. Copy the table on the next slide and complete it as you collect your results. Common Solutions and their pH Numbers Name of Solution Q1. Q2. Q3. Colour with universal indicator pH number Which of the solutions were acid? Which of the solutions were alkali? Which of the solutions were neutral? Acid, Alkali or Neutral Make your own indicator… Collect the experiment card Summary Acid solutions have a pH range of 1 to 6 Alkaline solutions have a pH range of 8 to 14 Neutral solutions have a pH of 7 Universal indicator can be used to test the pH of a substance We use a scale called the pH scale. You can now collect homework four