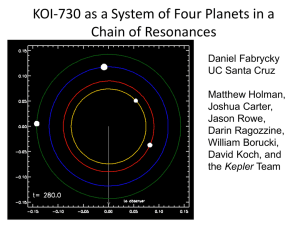
A B - Springer Static Content Server
advertisement

Sample preparation protocol produces smaller amounts of oxidized filgrastim. During the first two steps, immobilized metal affinity chromatography (IMAC) and tag cleavage, the pH of solutions containing labeled Filgrastim was kept above 7.0 in the absence of reducing agents to maintain the disulfide bridges. Such conditions were not optimal since they favored oxidation of the free sulfydryl on Cys-17. Analysis of the 2D-1H,15N-HSQC spectrum (Fig.1) indicates extra resonances belonging to one or several minor species. The SDS-PAGE analysis (Supplemental Fig. 1) of the sample did not show the presence of extra bands, such as a severely truncated protein, with intensities that correlated with the intensities of the extra resonances. We hypothesized that a fraction of the sample may be oxidized at Cys-17. In order to investigate this, we subjected the sample to mass spectrometry analysis and we carried out a modified purification protocol aimed at shortening the time, from three days down to a few hours, where the protein is exposed to high pH (greater than 7.0), thus being exposed to oxidation conditions. Therefore, as soon as the on-column refolding and IMAC purification were completed, the pH of the sample was lowered to pH 4.0. The poly-histidine tag was thus left in place, because its removal can only be done at pH 8.0. Analysis by NMR (Supplemental Fig. 2) showed that the intensity of the extra resonances was diminished. This observation thus suggested that the minor species observed resulted from oxidation. The MS analysis of peptide fragments resulting from chymotrypsin digestion of filgrastim followed by capping, or not, of the free cysteines with iodoacetamide indicated that Cys-74 was doubly oxidized (+32 a.m.u.) corresponding to the addition of two oxygen atoms on the sulfur atom of the side-chain), contrary to our expectations. Supplemental Figure 1: SDS-PAGE analysis of the 15N-Met-G-CSF sample did not show the presence of extra bands, such as a severely truncated protein, with intensities that correlated with the intensities of the extra resonances. Supplemental Figure 2: Overlay of two-dimensional NMR 1H-15N-HSQC of 15N-Met-GCSF. The spectrum of 15N-Met-GCSF (blue) shows a number of extra resonances showed with arrows that are attenuated when a modified purification protocol is used. The red spectrum corresponds to 15N-polyHIS-Met-GCSF obtained with the same purification protocol but interrupted after the on-column refolding. The green spectrum was recorded on 15N-Met-GCSF where the fraction collection after the nickel column was performed under argon and the pH of fractions was readily lowered to 4.0, prior to NMR sample preparation, i.e. buffer exchange and concentration. Note that the poly histidine tagged protein has and extra 23 residues. Signals at 9.4 and 120 ppm (marked by arrows) are two of the extra resonances attributed to the oxidized proteins by comparison with Neupogen spectra, and are in a well-resolved section of the spectrum. The intensities of these resonances changed with the limitation of oxygen exposure provided the modified purification protocol. These resonances were good indicators on the level of extra oxidation of the protein. Supplemental Figure 3: Effects of temperature on the two-dimensional NMR 1H-15N-HSQC spectrum of 15N-Met-GCSF at 25oC (violet); 27.5oC (blue); 30oC (cyan); 32.5oC (green); 35oC (orange); 37oC (red). Supplemental Figure 4: A) Effects of pH variation on the two-dimensional NMR 1H-15N-HSQC spectra of 15N-Met-GCSF. B) Overlay of the aromatic regions of the 2D 1H-13C-HSQC, recorded at four pH values. The spectrum collected at pH 1.5 results from brining back the sample from pH 6.3 where precipitation was taking place. Therefore it is noisy due to the loss of protein. At this low pH, the protein fold may also be less stable; the His H2 protons are slightly shifted upfield. A B Supplemental Figure 5: Effects of ionic strength on the two-dimensional NMR 1H-15N-HSQC spectra of 15N-Met-GCSF. Supplemental Figure 6: Structure of the receptor-bound filgrastim (PDB ID code 2D9Q) showing the three mutations (green spheres). The alanine next to the disulfide bond is A37. A29 and A30 are at the edge of the receptor-binding surface. Supplemental Figure 7: Overlays of spectra of A-G mutants A29G, A30G, A37G, and A29-30G. Residues near the mutation in the primary sequence are circled for ease of analysis. Supplemental Fig 8: Overlay of 1mM Neupogen (see text), ~0.3 mM EDQM-CRS for filgrastim and 0.3 mM 15N-filgrastim. Note the red streak at 7.0 ppm and ~117 ppm are artifacts/impurities that appeared after purification.