DNA replication
advertisement

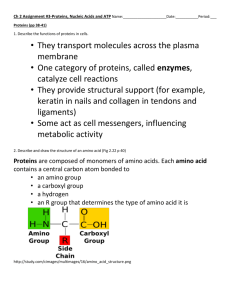
AP Biology Final Review PPLau,Ph.D. 2014 HSHP Biochemistry 1. Substrate binds to enzymes by weak interaction not covalent bonds, H-bonds etc. 2. Amylase digests carbohydrates (starch) to simple sugars (glucose, fructose etc). Human have alpha amylase which digests starch. Cows have beta amylase that digests cellulose. 3. Rate of enzyme reaction= change of substrate with time, or change of product with time. The slope is the rate. 4. The initial rate is linear, and logistic afterwards due to substrate concentration decreases. 5. Bell shaped curves for Rate vs pH or Rate vs Temp. The decline is caused by enzyme denaturation (not degradation or dead) 6. More enzymes, higher the slope on the rate curve 7. NH2 is the amino group, COOH is the acidic group. 8. Primary structure for protein: the amino acids sequence, bound by covalent bonds, named peptide bonds. (AAn, formed by dehydration, losing n-1 water.) 9. Secondary structure caused by H-bonding intramolecularly ( helix and sheet) 10. Tertiary structure caused by H-bonds and S-S bonds intermolecularly. 11. quaternary structure caused by dimerization of the same peptides (two of the same bound together by vanderwall, H-bonds, S-S bond, anything except covalent); or trimers or tetramers. 12. Know the structure of the neutral fats: triglycerides (3 fatty acids bound by glycerol) high energy molecules b/c they have a lot of hydrogens. Phospholipids have two fatty acids and highly charged on the phosphate. They are in phospholipid bilayer (cell membrane). 13. Know the structure of steriod (fused rings): sex hormones, cholesterol etc. 14. Know the structure of ATP and last (outtermost) phosphate bonds are highenergy. Cell and trasport 1. know these organells: ribosomes, mitochodria, lysosomes, Golgi, rough and smooth ER (roung makes proteins; smooth makes lipids.) 2. Prokaryotes have no membraneous organells. Nucleus is one of them. Ribosome is not membraneous. Prokaryotes, also known as bacteria, have ribosomes. All cells have cell membranes. 3. Know Nucleolus which is a compartment inside the nucleus and make ribosomes and rRNA and all RNA. RNA are made inside the nucleus. 4. Bulk transport: phago (solid), pino( water), endo, exo-cytosis. 5. Mitochondria is for cell respiration only. 6. Chloroplast is for making only glucose. 7. Know your endosymbiosis theory: Circular DNA, rRNA, makes its own proteins, the same size as bacteria, 8. Nephron uses facilliated diffusion (no energy needed )and active transport (needs energy), regulated by hormones (ADH and aquaporins) 9. All pumps (proton, sodium, potassium) are active transports. 10. Gluose diffuses into cells by faciliated diffusion. 11. Chemiosmosis involves the movement of H+ across a enzyme complex called ATP synthase (turbine). The force move the H+ is due to a concentration gradient and an elctrochemical gradient. H+ has a possitive charge and the outside of the thylakoid is negative. 12. Osmosis is the movement of water across a membrane due to differences in solute concentration or water potential. DNA replication 1. The goal is to make identical DNA. Double the amount. 2. DNA polymerase is an enzyme that base pairs a nucleotide with a nucleotide that is complementary to it. (A to T, G to C) 3. Helicases are enzymes that unwind the DNA molecule into single strands of DNA 4. During DNA replication hydrogen bonds between nitrogenous bases are broken not the sugar-phosphate bond of each strand. 5. A phosphodiester bond is a bond between two nucleotides linking 2 deoxyribose. It connects a phosphate group of one nucleotide and connects to the next nucleotide at the three prime end. 6. No enzymes are needed to form the hydrogen bonds as they form when the DNA polymerase pairs the DNA nucleotides. 7. DNA replication is semi-conservative: a new strand is made on top of a old strand. It is also anti-parallel. 8. Know your repliaton fork. 9. 32P radioactive labels DNA (phosphate), 35S labels proteins. 10. A mutation is a change in the DNA sequence of a gene 11. DNA doubles in S phase. (part of interphase) 12. nuclear membrane breaks down during prophase of mitosis. 13. apical meristem is where mitosis is occurring. 14. Mitosis is to make identical cells 15. Cancer cells are cells that have uncontrolled mitosis or cell division. Photosynthesis 1. Know the lab: bromophenol blue is bleached by oxygen. So we can use it as an indicator for photosynthesis (oxgygen released). 2. LDR (light dependent reaction) releases oxygen (byproduct) and the hotties NADPH and ATP. The hotties are used to power the Calvin cycle. The oxygen comes from splitting the water. Chemiosmosis happens with the H+ built up and diffuse through the protein channel of ATP synthase to make ATP. 3. Cyclic electron flow only happens in Photosystem I and makes more ATP but not NADPH. No oxygen is produced either. 4. The pigments are chlorophylls (absorb red and blue lights, not green) and carotennoids (only blue ight). Electron from the Mg in the porphyrin jumps to higher excited state and was accepted in the reaction center. The electrons released from the split of water supplement the loss of that electron. 5. Calvin cycle: a carbon fixation cyclic process: CO2 bond with the two timer (RubP) which is 5-C, with the enzyme Rubisco to make a 6 C compound which splits right away into two 3-C compounds. Wtih ATP and NADPH, the 3-C compound turns into 6 G-3-P. One G-3-P (or called PGAL) leaves the chloroplast and form dimer to make one glucose (6C compound). The rest of the 5 cycles back to become RuBP again with ATP. 6. Photorespiration: Stomata close during hot and sunny day to conserve water. Thereby short of CO2 and a rise of O2. All the RuBP are converted to respiration because the Rubisco enzyme is not specific to CO2. O2 binds to RuBP and losing the 5-C compound and therefore energy. Photorespiration does not make ATP unlike respiration. 7. C4 adaptation (spatial): CO2 binds to PEP ( a 3 C compound) in the mesophyll and form a 4 C compound. As it reaches the bundle sheath cells, it breaks down to CO2 and a pyruvate ( 3C compound). The CO2 is released and goes into Calvin cycle. 8. CAM adaptation (temporal): CO2 binds to organic acid CAM at night and release CO2 at night. Then CO2 can be used at night for the Calvin cycle to make sugar. Respiration 1. Study the comparison of Chemiosmosis in chloroplasts and mitochondria. Text p188. 2. All cells have the biochemical pathway glycolysis, which would make it a very ancient biochemical pathway. Not all cells have the Krebs cycle and the electron transport chain. In terms of evolution, glycolysis (anaerobic respiartion) is probably more ancient than aerobic respriation. ( Glycolysis occurs in the cytoplasm ). Glycolysis: splitting glucose into two pyruvate (3C compound) in the cytosol. 3. Fermentation only nets two molecules of ATP compared to approximately 36 in aerobic respiration, which oxidizes glucose to 6 molecules of carbon dioxide and 6 molecules of water 4. Oxygen is used in cellular respiration (metabolism) as the final electron acceptor at the end of the electron transport chain. 5. The electrons originate from either NADH or FADH in the electron transport chain 6. the pH is lowered and a proton gradient is established where there are more electrons in the outer compartment of the membrane than in the inner compartment. The outer compartment is positive relative to the inner compartment. 7. Aerobic respiration is considered to be more efficient as it results in many more molecules of ATP (approximately 36 ATP) than fermentation (2 ATP). 8. -Carbon dioxide is formed in the Kreb’s cycle. 9. -Carbon dioxide does not combine with lactic acid to form pyruvic acid. 10. -Oxygen does not catalyze the glycolysis reaction 11. The more hydrogen an organic molecule has, in general, the more energy it will contain. 12. In general the smaller the endothermic animal, the greater their need for energy and increased cellular respiration. 13. The rattlesnake will have the slowest rate of respiration because it is a ectotherm and does not produce heat to maintain a constant body temperature. 14. Negative feedback occurs when one of the products of a biochemical pathway is an inhibitor or negative modulator for one of enzymes in the pathway. Phylogeny or Taxonomy (adaptations of animals and plants) 1. Study vertebrate and invertebrate phylogeny trees from the Brown book, including p 193. 2. Some simple facts: Arthropods have the three germ layers, bilateral symmetry, a true coelom and an exoskeleton; Flatworms have the three germ layers, bilateral symmetry; however they do not have any sort of body cavity of coelom; earthworms have closed circulatory system and nephridia; fungi are heterotrophs, plants are autotrophs 3. trachophytes are plants with vascular tissues 4. Nitrogenous wastes are released as ammonia, urea, or uric acid. Ammonia and urea are soluble in water, but uric acid is not. Of the three, ammonia is the most 5. 6. 7. 8. 9. 10. 11. 12. 13. toxic and needs to be excreted quickly. Freshwater fish quite often excrete nitrogenous waste as ammonia. Urea is less toxic but still needs to be excreted with water (forming urine). Many marine and terrestrial animals excrete urea. Uric acid is the least toxic of the three. It is insoluble in water and is excreted as solid crystals. Reptiles, birds and insects excrete uric acid. For birds and reptiles this is beneficial because it is insoluble it can be stored in an egg in a special membrane (allantois) as nitrogenous waste and not be toxic to the embryo. Also birds need to be as light as possible so using uric acid as the nitrogenous means there is no water component need to flush it out. Birds do not urinate. The white material found in bird dropping is the uric acid Characteristics of the various kingdoms: extreme environment (high temp, salt) is a derived characteristic for Archaebacteria. Fern, unlike angiosperms and gymnosperms do not produce seeds. Gymnosperms are tracheophytes that produce naked seeds meaning they do not develop inside a chamber or ovary. As a result, they do not develop fruit. Angiosperm produce fruits. Cnidarian include jellyfish and hydra. The jelly fish is like an inverted hydra. Chordates have notochords. Echinodermata includes sea stars and brittle stars. They have radial symmetry and are sessile as adults. Sponges or Porifera have no coelom and are asymmetrical. Annelids are segmented worms and are also protostomes Botany 1. Endosperm provides the food for a growing embryo. The endosperm was created when it was fertilized by a second sperm cell. 2. Flowers are adaptations to ensure pollination and ultimately fertilization. 3. Fruit attracts animals to eat the fruit with the seed. 4. The female gametophyte in plants is produced in a protective jacket of cells called the archegonia and in higher plants it may be replaced by the ovule found in the ovary or carpel. 5. Xylem and phloem tissue are made as primary tissue by the apical meristem. It is also made as secondary tissue by the vascular cambium. 6. The driving force of the movement of water through xylem is transpiration pull 7. Guttation is caused by root pressure. Root pressure may contribute to the push of water up small, herbaceous plants but cannot supply enough to push the water up a 15-meter tree 8. Parenchyma cells have the ability to divide and give rise to other types of tissue. 9. Sclerenchyma and xylem cells are dead at maturity. Both function in support. 10. Collenchyma tissue is alive at maturity and functions in support. Collenchyma cells have thicker primary cells walls than parenchyma. They cells form cylinders and are found the midrib of leaves. Collenchyma tissue does not divide. 11. Sieve cells are found in phloem tissues, and even though they do not have a nucleus, they are metabolically active. 12. Meiosis occurs in the anther to produce microspores and in the ovule to produce megaspores. 13. Anthocyanin is a plant pigment and not a hormone or plant-growth regulator 14. Ethylene is a simple gas that is a hormone for ripening 15. Indoleacetic acid is another name for the hormone called auxin, for elongation. 16. Abscisic acid is the hormone causes aging in a plant. 17. Cytokinnin is important is important in regulating cell division in roots and shoots 18. It is the length of the night that is responsible for short-day plants; length of the night that triggers flowering not the length of the day. 19. The acronym to help you remember how water moves up xylem tubes is the T.A.C.T theory. (T.ranspiration pull A.dehsion C.ohesion T.ension). 20. Phloem is responsible for the movement of water and organic nutrients (mainly sugars) throughout the plant. This process is called translocation. 21. auxins cause cell elongation, and that gibberellins cause an increase in cell division. 22. The order of seed germination 23. Fruit is derived from tissues of an ovary 24. Auxins activate proton pumps which causes the lowering of pH that activates enzymes called expansins. 25. Many plant cells are terminal cells and do have the ability to reproduce (replicate as in mitosis). Plant cells that have the ability to reproduce are called meristematic cells. There are two types of meristematic tissue. The first is the apical meristem and it is located in the tips of roots and shoots and produces primary tissues. This causes the plant to increase in length. The second type of meristematic tissue is lateral meristem which produces secondary tissue and causes the plant to increase in girth. There are two types of lateral meristem which is the vascular cambium and the cork cambium. The vascular cambium produces the secondary vascular tissue of secondary xylem and secondary phloem. Cork cambium produces cork cells which will make up the outer bark or periderm. 26. The Carsparian strip is a waterproof band of material found on endodermal cells 27. Transpiration is the loss of water vapor through the stomata of the leaves. 28. The three parts to a seed is the seed coat derived from the ovule, the embryo, and the food. The food is called endosperm and is triploid (3n) as a result of double fertilization. 29. Water moves from high water potential to low water potential. (Osmosis) 30. learn the calculations of water potential in osmosis lab. Body Systems (study the brown book or blue book) a) Circulatory 1. The lungs of the frog are very primitive and simply amount to two sacs that provide a surface for gas exchange compared to human lungs, which are intricately subdivided into very small chambers for gas exchange. To supplement the lungs, the skin of the frog and the lining of its mouth also provide a surface area for gas exchange 2. During fetal circulation, the blood does not pass to the lungs for gas exchange. There is an opening between the two atria called the foramen ovale. The blood flows from the right atrium to the left atrium so that it does not flow to the lungs. Gas exchange occurs in the placenta between the fetal blood and the maternal blood. This opening closes once the baby is born the the blood goes from the right atrium to the right ventricle and then to the lungs for gas exchange. 3. The reaction Hb(O2) 4 -> Hb + 4O2 happens is when tissues or cells need oxygen. This release of oxygen happens in response to production of carbon dioxide as cells produce ATP in cellular respiration. An increase in oxygen would indicate that the cells did not need oxygen, and the hemoglobin would not release the oxygen. PH tunrs acidic due to CO2 lower the affinity of hemoglobin for oxygen and oxgygen comes off the complex: Bohr Shift (p243 of the brown book or blue book) 4. If one is exercising strenuously, then there will be an increase in temperature. In addition because of an increase demand in energy, there will be an increase in cellular respiration. An increase in cellular respiration will produce more carbon dioxide. The majority of carbon dioxide is carried in the blood as carbonic acid. This increases the acidity of the blood. 5. One requirement of gas exchange is that the gas must be in solution and there for the gas exchange membrane must kept moist. 6. Carbon dioxde dissolves into the blood plasma. Over 90% of the carbon dioxide moves into the red blood cell where it is easily converted to carbonic acid 7. The counter-current exchange system found in the respiratory system of fish is the movement of water in the gills of fish in the opposite direction of the blood flow in the gills. This facillitates maximum pick-up of the gas. 8. grasshopper has an open circulatory system 9. The osmostic pressure exceeds the blood pressure at the venous end of a capillary 10. right atrium is the chamber in which the pacemaker (S-A node) is located, right atrium is the chamber that receives blood from the lungs 11. The right ventricle is the chamber that pumps blood to the lungs, The left ventricle is the largest chamber of the heart. 12. The horizontal line represents osmotic pressure where the change of weight after diffusion is zero in the dialysis bags of the sucrose diffusion experiment. B) Digestion and Excretion system 1. The nephron is the filtering unit of the kidney. It works by filtering the blood in the glomerulus. Once the filtrate is in the nephron, nutrients like glucose and amino acids are reabsorbed into a capillary bed. Then there certain substances are secreted into the nephron. This is called tubular secretion. 2. When animals (especially vertebrates) consumer proteins, the proteins are digested (catabolism) and broken down to amino acids. Once the amino acids are absorbed by the blood stream, they are delivered to the liver. If the amino acids are needed for energy, the amine group (NH2) is removed (catabolism) and turns into amonia and the liver will covert that to urea. (see Taxonomy 4. Nitrogenous wastes are released as ammonia, urea, or uric acid.) 3. If there is less energy available due to a decrease in the rate of cellular respiration when temperature is decreased, then it follows that the heart rate would also decrease. 4. Mammals are endotherms, which means their physiology regulates their temperature so that it stays rather constant. A decrease in temperature will cause number of physiological events to occur such as 1)Blood vessels close to the surface of the skin to constrict to conserve heat, not the blood vessels in the deep muslces. 2) A release of thyroxine will increase the metabolic rate and increase heat production. 3)Increased secretion of epinephrine increases the amount sugar release into the circulatory system to give energy to shivering muslces. 5. Blood cells and plasma proteins are too large to pass into Bowman’s capsule and do not enter the nephron. The filtrate that enters Bowman’s capsule is essentially, plasma minus the plasma proteins. It contains materials like glucose, urea, salts, amino acids vitamins and other molecules. The beneficial material like glucose and the amino acids are reabsorbed by the proximal convoluted tubule. 6. Essential amino acids are those amino acids that cannot be synthesized by the organisms. We have 8. 7. Protein digestion begins in the stomach with pepsin but like all other nutrients, the amino acids are absorbed in the small intestine 8. Gram for gram, fats or lipids have more calories than either proteins or carbohydrates. 9. Filtering into the Bowman’s capsule by the hydrostatic pressure arising from the heart beat. 10. Because animal cells do not have cell walls to prevent them from bursting, it is important that interstitial fluid be either isotonic or hypertonic. If the fluid is hypotonic, the cells will burst. If the fluid is too hypertonic, the cells will loose too much water and die. 11. The loop of Henle is longer in animals found living in the desert and is shorter in animals living in areas where water is plentiful. 12. The digestive tract of the omnivore was about three times longer than their body length. The digestive tract of the herbivore was about ten times longer than their body length. Plant matter is much more difficult to digest than annimal matter C) Hormones and development 1. The ovary is where primary oocytes are located. 2. Placenta: fetal blood is kept in capillary beds. This prevents the mixing of the maternal and fetal blood unless the capillary bed is broken open. This arrangement allows for gas exchange between maternal and fetal blood and a way for the fetus to get rid of nitrogenous waste products. The formation of the placenta originates from both embryonic and maternal tissues. Placenta produces prostaglandins, which are hormones and help stimulate with contractions of the uterus during childbirth. 3. Antidiuretic hormone (ADH) is produced by the pituitary gland and increases the permeability of the collecting tubule. As the filtrate moves through the collecting tubule, it causes water to move into the renal tissues into the capillary bed. The net effect of this will concentrate the urine and retains more water for the organism. Aquaporins play a major role in reabsorption of water in the tubule through the action of ADH. 4. The acrosome as seen is at the tip of the head of the sperm cell. It contains hydrolytic enzymes that aid in the sperm penetrating the egg. 5. The uterine lining thickens for the implantation of the embryo after fertilization occurs trhough the action of estrogen and progestrin. 6. Fertilization in humans normally occurs in the oviducts. 7. One major difference between plant embryonic development and animal embryonic development is a major movement of cells in animal development. There are two major movement of cells. The first movement of cells is called gastulation forming the 3 germ layers: ecto endo and mesoderm. The second major movement of the cells is neurulation which results in the establishment of the neural tube. 8. Progesterone is secreted by the ovaries. It promotes the uterine lining growth and is responsible it maintenance during the pregnancy; regulated by FSH and LH. 9. Human identical twins develop when the embryo splits and each portion continues to develop. Usually this is at the two-cell stage. In order to identical twins, the twins have the identical DNA. So this must happen after fertilization occurs. 10. The fish does not lay amniotic eggs and therefore does not have an amnion. Aminion is a water sac for shock absorption. Fish don’t need them. They have external fertilizaton. 11. Insulin lowers blood glucose levels in the blood a number of ways. One of which is the conversion of glucose to glycogen in the liver and muscles. 12. The release of adrenalin from the adrenal glands causes the “fight or flight response”. This response includes an increase in blood flow to the skeletal muscles and skin 13. Animal hormones fall into one of three categories-modified amino acids (glutamine), sterioids (sex hormones), or polypeptide chains (insulin as peptide) 14. The parathyroid gland produced parathyroid hormone (PTH) which regulates calcium and phosphorous in the blood along with calcitonin produced from the thyroid gland. 15. The placenta is used to obtain nutrients from the mother’s blood for the fetus. In additon the placenta is used for gas exchange. The fetus has its own heart to circulate its own blood. 16. Ectoderm gives rise to the nervous tissue, (brain, sense organ etc);Endoderm: endothelial lining of vessels; mesoderm: muscles, bones excretory, reproductive (p1007 textbook). D) Immune system 1. Substances that are foreign to an organism and stimulate an immune response are antigens. Antigens in can be proteins or carbohydrates. 2. Humoral immunity involves the production of antibodies in response to the presence of specific antigen. An antibody is a protein secreted by plasma cells (T-lymphocytes) and can chemically combine with a particular antigen to immobilize the antigen so that it can no longer cause disease. 3. Interferon is a substance secreted as a part of the nonspecific immune response. It induces neighboring cells to make substances that inhibit viral reproduction. Therefore it makes sense that if interferon is effective against certain forms of cancer, then viruses may cause certain forms of cancer. 4. HIV virus attacks white blood cells, more specifically help T-cells. The protein that the HIV virus attacks is a protein called CD4. 5. The substance that causes the inflammatory response is histamine 6. T-cells are involved in the cell-mediated response producing cytotoxic T-cells. Bcells and plasma cells are involved in the humoral response. 7. All blood cells originate in the bone marrow. B-cells mature in the bone marrow but T-cells mature in the thymus gland. 8. An antigentic determinant is that part of the antigent that elicits the production of a certain antibody. 9. When the immune system is exposed to a disease for the first time, as the cell mediated and humoral response is initiated, there a memory cells made. The memory cells are used, they quickly produce B-lymphocytes and T-lymphocytes to start the immune response. This is much faster then the primary immune response and most often the person does not even know that they have been exposed to that disease. 10. Plasma cells are selected B-lymphocytes involved in the production of antibodies in the immune response. 11. The function of helper-T-cells is to select the (right antigen) specific B-cell and the specific Tc (cytotoxic T) cells for proliferation (multiplying). Tc cells kill infected cells and B cells make antibodies (circulating in the blood, therefore humoral). 12. The inflammatory response increases permeability of the blood vessels in order for the leukocytes and other proteins 13. Blood donors: A B donor has B antigens on the surface of their erythrocytes and an O recipient does not and therefore will make antibodies againt the B antigen. 14. The cell-mediated immunity results in infected cells being lysed by cytotoxic Tcells because they have MCH receptor sites. 15. The antigen presenting cell first engulfs the pathogen, then destroys the antigen and combines it with a MHC protein. This is done by phagocytosis by a macrophage. The MHC protein with its antigen is then inserted into the plasma membrane of the macrophage cell. It then looks for a T-cell that has the correct receptor site for the MHC protein and antigen. (study the brown or text book) E) Nervous and muscle systems: 1. There are ion gradients that exist along a neuron. In general there are more positive ions outside the neuron because of a gradient of Na+ ions and on the inside of the membranes there are more K+ ions but there are also proteins that have net negative charge. This causes the outside of the neuron to be positive relative to the inside of the neuron, which is negative. The interior of the neuron is a -70 mV when compared to the outside of the neuron. This is called the resting potential. It is because of the existence of sodium potassium pumps, that the resting potential exists. 2. Increase blood flow to the skin will lower the body temperature of a mammal because at the surface the air will remove the heat. If the mammal has sweat glands, the water vapor of the sweat will carry the heat away. Water absorbs considerable heat (more than air) when it evaporates. 3. In the spinal cord there is an association neuron (interneuron) which sends the signal to both the brain via other interneurons and innervates the motor neuron. The motor neuron innervates the effector (muscle) to contract. 4. The “all-or-none” response refers to a single neuron. In order for a neuron to “fire” (send an impulse down the neuron), There is a change in the voltage across the membrane. This is accomplished by sodium ions moving across the membrane. It turns out there is a threshold level or critical voltage (-55mv) that must be reached. 5. Smooth muscle is found in the digestive tract, urinary bladder, blood vessels, and other internal organs. Autonomic nerves stimulate these muscles. 6. Neurotransmitters must be broken down or the post synaptic neuron will continue to send an impulse down the neuron. Cholinesterase or acetylcholinesterase is an enzyme that destroys acetylcholine so that the action potential stops. 7. The sympathetic nervous system causes the flight or fight response. This causes things like dilation of the pupils, inhibits salivary glands, relaxes bronchi in the lungs, accelerates the heart rate, inhibits digestion, inhibits emptying of the bladder and lastly cause the liver to release glucose. 8. The corpus collosum contains a thick band of axons that allows the left hemisphere to communicate with the right hemisphere in the cerebrum. 9. The medulla oblongata is the part of the brain stem and functions in the transfer of information between the peripheral nervous system and the midbrain and the forebrain. It also aids in large movements such a walking, running and swimming. 10. Neurotransmitters are found in small membrane-bound vessicles and are molecules that diffuse from the presynaptic neuron to the postsynaptic neutron. There is a small space between these two neurons called the synaptic cleft and the movement of the neurotransmitters is passive diffusion. 11. Glycogen (a polysaccaride of glucose), an immediate source of glucose in muscle cells, decreases when a person strenously exercises as it is converted to ATP. 12. Glial cells are associated with neurons. They wrap themselves around the neurons and form the myelin sheath. 13. Rod and cones are cells that can detect wavelengths of light. The rods are not sensitive to color 14. muscle contraction needs calcium. EDTA stops the binding of calcium and prevents the muscle from contracting but does not interfer with acetylcholine which starts the action potential. 15. Acetylcholine is the neurotransmitter that attaches to receptor sites on the membrane of the muscle. Ecology 1. four trophic levels: the producers, primary consumers, secondary consumers and tertiary consumers. 2. Decomposers are usually prokaryotes and fungi 3. the base (lowest trophic level) is the producer level. A producer is an organism that can fix carbon dioxide into an organic compound like sugars and starches. 4. Competitive exclusion occurs most often results because of interspecifiic competition. 5. the concept of competitive exclusion is the idea that if two species are competing for the same limited resource, one species will be more efficient at obtaining the resource than the other. This will effectively eliminate one or the other species by extinction or emigration. 6. A density-dependent limiting factor is one that slows down population growth as the population increases in size. 7. Nitrogen fixation is a biochemical pathway that is found in certain prokaryotic cells (bacteria) and only prokaryotic cells. It takes atmospheric nitrogen N2 and reduces it to ammonia. It is an endergonic reaction and requires 16 molecules of ATP. N2 + 18 e- + 16 ATP -> 2 NH3 + H2 + 16 ADP + 16 Pi 8. Heterotrophs need organic molecules as a source for energy and building blocks. Heterotrophs cannot make their own food and must get their energy from the environment. 9. Commensalism is type of symbiosis, in which one organism benefits and the other organism is neither harms or does it benefit. Predation is an association in which one organism benefits, and the other is harmed, as it is hunted and eaten. Parasitism is a type of symbiosis in which one organism benefits and the other organism is harmed. Mutualism is a type of symbiosis in which both organisms benefits. Competition happens when two organisms are using the same resource and are living in the same area 10. One major difference between the forest ecosystem and the grassland is the amount of annual precipitation. The amount of annual precipitation varies from 30-100 cm. Where as in the temperate deciduous forest, the annual precipitation varies from 70-over 200 cm. 11. Bacteria secrete exotoxins. They are usually protein and are usually very toxic. Diseases caused by exotoxins include botulism, cholera, anthrax, and the plague. 12. Pollution: Biological magnification is process in which certain compounds become more concentrated as it moves to higher trophic levels in the food chain. That being the case, the vulture is at the top of the food chain and it would be more concentrated in the vulture than in the other animals. 13. Net primary productivity, which is the total amount of carbon fixed by photosynthesis over a given time period minus the amount oxidized during cellular respiration. If the rate of carbon fixation is greater than the rate of cellular respiration, then the biomass of the ecosystem will increase. If the rate of cellular respiration of greater than the rate of photosynthesis, then the biomass is decreasing. (one makes and one burns) 14. Ozone is composed of oxygen or O3 molecules. The biochemical pathway that is responsible for putting oxygen into the atmosphere is photosynthesis. 15. Natural eutrophication is the process by which lakes gradually age and become productive. This usually takes many years to achieve. This process can increase by pollution. The pollution is in the form of adding nutrient enrichment for plants and algae. The pollution comes in the form of nitrogen and phosphorous from sewage and water run-off from places like golf courses. Eutrophication causes algal blooms. While at first this seems like positive thing it is not. Because the alage will die and then there is a “bloom” of decomposers. The decomposers will remove oxygen from the water resulting in fish kills. 16. As energy is moved from one trophic level to the next a certain amount of energy is not transferred. There are three reasons why this is the case. 17. Interspecific competition occurs when two similar species in the same location are competing for the same resource. If the niches overlap too much, then it could result in restricting the range of the species so they do not overlap, or the migration of one species to another place. 18. Trial and error learning is a animal learning how to avoid the distasteful response. 19. A characteristic of a fast-moving stream includes high levels of dissolved oxygen. Because the water is being constantly stirred up, it increases the amount of oxygen able to dissolve in the water. 20. Denitrification is a biochemical pathway in which anaerobic bacteria are able to use nitrate (NO3) in their respiration instead of oxygen and as product nitrogen gas (N2) is released back to the atmosphere. 21. When using pesticides, it is possible that a number of pests may contain a gene that makes is resistant to the effects of the pesticides. While the use of the pesticide may temporarily reduce the number of pests, there will be a few that survive and are resistant to the pesticide. 22. If a population of predators eliminates the population of prey, then the population of predators will be eliminated for lack of food. 23. Below is a generalized list of characteristics of r-strategists versus K-strategists. characteristics # of offspring # reproductive type of population growth pattern # of time needed investment of parental care r-strategists many usually one boom-bust K-strategists just a few Several episodes logistic growth short amount of time none takes years to mature will take care of young life span short longer- even years body size usually small usually larger 24. producers are either phototropic or chemotropic in the oceans. 25. Biomes: The tundra is characterized by mainly herbacious grasses, mosses and shrubs. There may be a few dwarf trees. Also there is a permanently layer of frozen soil (perma frost) which gives rise standing pools of water in the short summers. The taiga is characterized by long winters with plenty of snowfall and conifers (gymnosperms). The desert is characterized by less than 10 inches of precipitation per year. The temperature is variable. The extreme variation is 50oC during the day in the summer and -30oC during the night in the winter. 26. In a population that is in equilibrium, it will fluctuate around the carrying capacity depending on factors interacting with ecosystem. Things, like weather and availability of food, can have effects on the population and make it fluctuate around the carrying capacity. 27. exponential growth: The line curves upward in this part of the graph going left to right 28. Ecological succession is the process that occurs after the environment has been disrupted usually from some natural disaster like fire, volcanoes or hurricanes. It is the transition that takes place to climax community. Evolution and hardy weinberg 1. sympatric crickets are those living in the same area. So if two similar species of crickets are living in the same area, then there needs to be some way that the can recognize one another so there are no mating mistakes. Difference in cricket calls is an example of behavioral isolation alerting potential mates to their unique song. 2. Genetic drift is a change in the population’s gene pool due to chance. This can happen when the population is very small and the loss of genes can significantly alter allelic frequencies. 3. Emigration and immigration can alter allelic frequencies but if the population is large, then the impact on allelic frequencies is not significant. 4. Mutations provide the raw material for evolution but do not cause genetic drift. 5. Convergent evolution occurs when two unrelated groups of organisms that are exposed to the same selection pressures or environment. This causes the two groups the have similar traits but not due to the fact they are closely related. 6. Divergent evolution is two closely related groups that are different from one another due to different selection pressures or competition. 7. A species is a group of interbreeding organisms that produce viable, fertile offspring in nature. So a new species is formed from another group when they no longer can interbreed under natural conditions with other closely related organisms. 8. Homologous structures are structures found on different species that may vary in their function but these structures maybe closely related based on evolutionary evidence of a common ancestor. 9. Sympatric speciation is the formation of a new species without geographic isolation. 10. According to Hardy-Weinberg equilibrium, there is an equation which depicts the genotypic ratios. p2 + 2pq + q2 =1 where p2 =WW, 2pq = Ww q2 =ww (all % phenotypes) p and q are allelle frequencies. 11. The speciation of the finches on the Galapoagos Islands is due to allopatric speciation. 12. gene flow is the movement of alleles from one population to another population due to migration of individuals from one group to another group WITHIN the same species. 13. Bottleneck effect is results from genetic drift or significant changes in the gene pool due to chance because the population is very small. Usually, the small size of the population is due to a natural disaster or human intervention. 14. Hybrid sterility results in a sterile offspring that is the result of a mating from two different species. 15. External fertilization is characteristic of vertebrates that live in water. Once fertilization occurs, the embryo is surrounded by water and desiccation is not a problem. 16. Sexual selection is a form of natural selection in which individuals with certain inherited traits are more likely than other individual to obtain more mates. 17. Reproductive isolation occurs when individuals from different species cannot successfully interbreed because of some biological barrier. This may include barriers like gametic isolation, developmental isolation, hybrid inviability, and hybrid sterility 18. Punctuated equilibrium is based on the fossil record in which there are long periods of stasis followed by a rapid evolution of new species. It is thought to be caused by some sort of natural disaster that starts the rapid speciation 19. Darwin did not know about DNA and how muttations in DNA can cause variatons in phenotype. It was before his time. 20. Directional selection occurs when one trait changes in one particular direction. 21. Stabilizing distribution means that there will be an increase of the most common phenotype because it is the most beneficial phenotype and has some sort of reproductive advantage 22. Paleontology is the study of life and evolution based on the fossil record. Protein Synthesis and Gene Expression 1. A plasmid is small circle of DNA found in prokaryotic cells. It is smaller than the bacterial chromosome. 2. During development, individual cells of the same organism begin to produce different proteins because specific genes are activated in the cell. This is called cell differentiation. 3. transcription is to makes mRNA from a DNA gene 4. An operon is found in prokaryotic cells. It is a series of genes found in close proximity to one another. They are controlled by repressor proteins. Trp operon is operated by negative feedback (the product, trp, activates the inactive repressor) to slow down synthesis; lac operon is positive feedback. (it inactivate the repressor so that more product will be synthesized) 5. Histones are found only in eukaryotic cells not prokaryotic cells. 6. Reverse transcription is using RNA as a template and making a DNA molecule. 7. Only about 10% of the human genome is transcribed and translated. 8. In eukaryotic cells unlike prokaryotic cells, genes for related function, are often located on different chromosomes. They do not share the same promoter and operateor regions. 9. Frame shift mutation: changing the subsequent genetic codes of the sequence is most detrimental. 10. Both DNA and RNA uses phosphate groups and neither one uses sulfate groups. 11. A codon consist three nucleotides that specify an amino acid. There are four nucleotides so 4 x 4 x 4 = 64 combinations or codons. 12. Transcription occurs in the nucleus; Proteins are made in the cytoplasm (translation); 13. mRNA is made in the nucleus and determines the sequence of amino acids of a protein made in the cytoplasm 14. GTP is the energy used during translation 15. The enzyme that connects the right amino acid to the specific t-RNA is aminoacyl-tRNA synthetase. There are 21 amino acids, therefore 21 of these enzymes. 16. The process of the ribosome moving from one tRNA to another during process of making a polypeptide chain is called translocation. 17. Learn to read the codon chart, from left to right. Genetics You need to study the LTF genetics review packet and x2 analysis. Labs Study the LTF 12 labs review packet. FRQ LTF ( for example, I copied what are on the LTF Biochemistry) 1) Water 2 lone pairs of electrons 2 Hydrogens �Due to the two lone pairs of electrons (represented as the two on top in the picture), the molecular shape of water is bent. �Though oxygen and hydrogen are bonded covalently, oxygen is highly electronegative in comparison to hydrogen. This unequal sharing or electrons results in water molecules being polar with slight negative and slight positive charges. �Hydrogen bonds: This polarity results in hydrogen bonding between H2O molecules. �Nearly all other characteristics of liquid water can be explained based on the information above. �Cohesion and surface tension: water “sticks to itself” due to H bonding �Adhesion: water sticks to other substances so long as H bonds are a possibility. �Capillary action: such as water traveling up a straw is due to adhesion (water is sticking to the sides) and cohesion (water pulls up more water molecules as it moves up the side). �High specific heat & high heat of vaporization: Remember that temperature is a measure of kinetic energy. Even though individual H bonds are considered weak, a water molecule must break free from many H bonds when increasing in temperature or changing phase to water vapor. �Universal solvent: Due to the polarity of water molecules, they are “good dissolvers” of all things polar. �Ice floats: Due to the crystalline structure of ice, it is less dense than water. Biological significance of Water �Due to the high specific heat of water, much energy is needed to raise the temperature of water. During the heat of day, a body can sweat to cool off. A little bit of sweat will result in the absorbance of a great amount of heat. When heat exits the body, the body is cooling. If water did not have a high specific heat, more water would be needed to cool the body increasing the likelihood of dehydration. For the same reasons, bodies of water would experience greater temperature swings from midnight to midday were it not for the high specific heat of water. It may be more challenging for organisms to maintain homeostasis in this environment. �Ironically, floating ice acts as an insulator to the water underneath. Lakes are less likely to freeze completely due to the fact that ice floats. �This list is not all inclusive (surface tension: water striders,etc.). 2 ) Carbon & Bonding �Remember that the name ending denotes the type of covalent bond (single: -ane, double: ene, triple: yne) �Carbon bonding is naturally covalent NOT ionic. �Carbon has 4 valence electrons resulting in 4 bonds (tetravalent). Recall that shape is important in biomolecules. Because C has 4 bonding sites and can bond with itself, essentially endless chains and shapes can be created. These shapes are important in enzymes, protein channels, etc. �Functional groups: Be able to identify �Hydroxyl (-OH) �Sulfhydryl (-SH) �Carboxyl (-COOH or -COO-) �Animo (-NH2 or NH3+) �Phosphate (-PO4) �Methyl (-CH3) �Isomers: An isomer is a rearrangement of a molecule’s components. The same number and kind of each atom is involved, but they are rearranged. �Structural isomer- relocation of atoms �Stereoisomer (enantomer)- mirror image. •The importance is that arrangement and shape matters: Lamino acids are biologically available, R- are not. Building of macromolecules �Metabolism is the elegant interplay of catabolic (breaking down) and anabolic (building up) reactions. �Polymers are made up of smaller units called monomers �Dehydration synthesis removes an –H from one monomer and an –OH from another to form a bond (H+OH� H2O). This bond is called a glycosidic bond in carbohydrates. �Hydrolysis is the “catabolic reverse” of dehydration synthesis. Water is split, H and OH are added, bonds of the polymer are broken. �a) Carbohydrates have an empirical formula of CH2O unless they are polysaccharides. For polysaccharides subtract an H2O for each bond. Example: A polymer of 6 glucose molecules would have 5 bonds created by dehydration synthesis. C30H60O30 - 5 H2O = C30H50O25 �Carbohydrates typically have an –ose ending (glucose, fructose, lactose…) �Functions of carbohydrates �Energy source (stored in the C-H bonds): Glucose, glycogen �Structural: cellulose �Cell recognition: glycoproteins �Carbohydrates may be monosaccharides (glucose, fructose), disaccharides (sucrose, lactose), or polysaccharides (starch, cellulose, glycogen) b) Lipids: Energy is contained in the C-H bonds of lipids �Lipids are hydrophobic. �Common example: triglyceride (glycerol molecule with 3 fatty acid tails) �Cholesterol is a lipid. While cholesterol is more likely to participate in arterial blockage, cholesterol is needed in membranes for fluidity control and also acts as the precursor to steroid hormones. Bonding �Saturated fats: Triglycerides with only single C-C bonds are called saturated. They are “saturated” with the maximum amount of hydrogen. Because the fatty acid tails are straight and uniform it is possible to pack many saturated fats into a small area making a solid. Saturated fats therefore tend to be solid at room temperature. Sticky, hydrophobic fats are still quite sticky when absorbed into the water-based bloodstream. It is thought that these fats are more likely to stick to artery walls; because of this, saturated fats are/were often referred to as “bad” fats. �Unsaturated fats: Triglycerides with C=C bonds are not completely saturated with hydrogen (for every C=C two hydrogens must be removed). These are called unsaturated fats. When C=C bonds are introduced, “kinks” are more likely to occur (esp. cis formation). This shape results in the inability of the lipids to pack together closely and because of this, unsaturated fats tend to be liquid at room temperature. Proteins -globular (enzymes, receptors, channels, etc.) -fibrous (collagen, keratin, actin & myosin, etc.) -peptides (signals)“–ase” ending denotes an enzyme, non-enzyme proteins often end with “-in” -globular (enzymes, receptors, channels, etc.) -fibrous (collagen, keratin, actin & myosin, etc.) -peptides (signals) �“–ase” ending denotes an enzyme, non-enzyme proteins often end with “-in” Protein structural levels �Primary structure is the order of the amino acids (coded for by DNA/RNA) �Secondary structure is the interaction of primary structure with itself forming hydrogen bonds within the same chain of amino acids. �Alpha helix �Beta pleated sheet �Tertiary structure is the interaction of secondary structures with other secondary structures within the same chain of amino acids. Disulfide bridges between cysteines are common for added strength & stability. �Quaternary structure is the introduction of additional amino acid chains. Essentially the tertiary structures of one polymer of amino acids interacting with another (hemoglobin is the most commonly cited example. Protein Structures and Denaturation shape is of paramount importance to proteins. Shape is determined based on the sequence of amino acids. One change in an amino acid can have a drastic effect (sickle cell anemia, cystic fibrosis, etc.) �Denaturation is when the shape of a protein irreversibly changes. �pH-A high concentration of H+ ions will certainly interfere with negatively charged “R” groups. Once this occurs the most stable form/shape of the protein may be different from what was originally intended. Low pH may also denature a protein. Ion concentration- High concentrations of a specific ion may denature enzymes through a scenario similar to pH. �High temperature- High temperature results in a shift in protein shape to a form that is more stable at that temperature. Low temperature does not typically denature a protein, enzyme activity at low temperature decreases due to decreased molecular collisions. Summary�Proteins have a “window of life” in which they function properly. They are able to maintain shape as long as the pH, ion concentrations, and temperature are not at extremes. Protein shape is due to the levels of protein structure which begin with amino acid sequences coded for by DNA. In short genetic diseases can be explained by the following flow chart: A change in the genetic code�possible change in the amino acid sequence (“possible” due to redundancy of the genetic code)�change in shape of the protein likely useless protein. Nucleic Acids (DNA and RNA) �DNA is a polymer the monomer of which is the nucleotide. A nucleotide consists of a phosphate, deoxyribose, and a nitrogenous base(A,T,G,C). RNA is similar with differences being: single stranded, U instead of T, riobose instead of deoxyribose. Notation of sugar 5’ and 3’- Students should be able to point out the 5’ and 3’ and realize that this is a man made mechanism equivalent to giving a molecule a right side up/upside down nomenclature Adenine is complementary to Thymine; Guanine is complementary to Cytosine. Note that the A and G are both “double ring structures” and that T and C are “single ring structures.” A and G are referred to as purines. T & C are pyrimidines. (Mnemonic: Purines = a,g…Pure as gold). Due to size constraints of the DNA backbone, a purine (big) must combine with a pyrimidine (small) in order to fit. Why can’t A:C?...Note that there are three sites for H bonds on G & C and only two on A & T. The only possible combination that due to size and bonding is A:T and G:C. DNA is stable. The charged DNA backbone (thanks to phosphate) and polar sugars will be stable in the water based fluid in the nucleus, the hydrophobic bases are shielded internally from H2O. This is of course reminiscent of the phospholipid bilayer. �The sugar phosphate backbone is joined via a phosphodiester bond. The joining of this backbone is accomplished via dehydration synthesis. �DNA has a major groove and a minor groove. Note the difference in groove when looking up at the pic vertically. The significance of the major groove comes later during transcription as transcription factors are thought to “scan” the major grooves before unzipping DNA. Summary of LTF 1 Functional groups �Hydroxyl (-OH) �Sulfhydryl (-SH) �Carboxyl (-COOH or -COO-) �Animo (-NH2 or NH3+) �Phosphate (-PO4) �Methyl (-CH3) Common Molecules �Ribose �Deoxyribose �Glucose �Polymers of glucose may be starch, chitin, glycogen, etc. depending on bonding arrangement. No need to be able to decipher between them. �Glycerol �Saturated fat �Unsaturated fat �Cholesterol �ATP �DNA components � Purine � Pyrimidine � Deoxyribose (including 5’ and 3’ carbon notation) � Phosphate � Bonds (hydrogen, phosphodiester, covalent) �Amino acid structure �Hemoglobin