Organic Reactions Worksheet answers 2014
advertisement

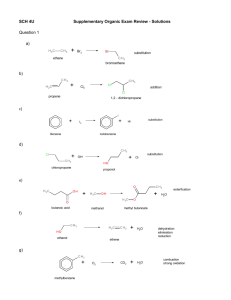
Organic Exam Review – Solutions Question 1 a) Br2 substitution ethane bromoethane b) Cl2 addition propene 1,2 - dichloropropane c) substitution HI I2 Benzene Iodobenzene d) OH- H2O chloropropane Cl_ reduction elimination propene e) esterfication H2O butanoic acid methyl butanoate methanol f) H2O ethanol dehydration elimination ethene g) O2 methylbenzene CO2 H2O combustion strong oxidation h) O O oxidizer oxidizer ethanol ethanal oxidation ethanoic acid i) H2O methanol ethanol dehydration methoxyethane j) NH3 H2O pentanamide pentanoic acid k) substitution H2 methane methanamine l) O H2 oxidizer oxidation propanone propan-2-ol Question 2 a) The reaction between benzene and 2 moles of chlorine gas. Cl + 2 Cl benzene AlCl 3 Cl +2H Cl meta-dichlorobenzene 1,3-dichlorobenzene Substitution Reaction b) The formation of hexan-2-ol from an alkene. Cl elimination dehydration + H2SO 4 H2O OH 100 C 1-hexene 2-hexanol hexan-2-ol hex-1-ene Addition Reaction c) The formation of pentane-2,2-diol. OH + H2SO4 2 H2O 1-pentyne pent-1-yne 100 C OH 2,2-pentanediol pentane-2,2-diol Addition Reaction d) The oxidation of hexan-3-ol with potassium dichromate. K 2Cr 2O 7 + H2O O OH 3-hexanone hexan-3-one 3-hexanol hexan-3-ol dichromate changes from orange to green Oxidation Reaction e) The reduction of butanal. Pt + O H2 butanal 101 MPa OH 1-butanol butan-1-ol Reduction Reaction (Addition Reaction) f) The reaction between trans-hex-2-ene with hydrogen bromide (to make two positional isomers). Br + trans-2-hexene trans-hex-2-ene + HBr hydrogen bromide 2-bromohexane Br 3-bromohexane Addition Reaction g) The formation of ethyl benzoate. O O OH benzoic acid + H2SO4 OH heat ethanol Condensation Reaction h) The formation of butan-2-ol. O ethyl benzoate + H2O + OH H2SO4 H2O 100 °C 2-butanol butan-2-ol 1-butene but-1-ene Addition Reaction -could also use 2-butene but-2-ene i) The reaction that would occur if ethyl propanoate was heated in the presence of water and acid. O O H2SO4 + H2O O ethyl propanoate j) OH heat + ethanol HO propanoic acid The reaction between 2 moles of water with pent-2-yne. OH OH H2SO4 +2 H2O 1-pentyne pent-2-yne 100 C OH 2,2-pentanediol + OH 3,3-pentanediol pentane-2,2-diol pentane-3,3-diol Addition Reaction k) The oxidation of butan-2-ol. O KMnO4 OH + H2O butanone 2-butanol butan-2-ol - KMnO4 changes from purple to brown - K2Cr2O7 will change from orange to green Oxidation Reaction l) The oxidation of butan-1-ol. KMnO4 OH 1-butanol butan-1-ol O + O KMnO4 H2O HO butanoic acid butanal - KMnO4 changes from purple to brown (K2Cr2O7 will change from orange to green) Oxidation Reaction m) Show the formation of but-2-ene from an alcohol. OH H SO 2 2-butanol butan-2-ol 4 100 °C 2-butene but-2-ene Elimination Reaction - also produce 1-butene but-1-ene + H2O + 2 H+ n) Show the reaction between methanol and butanoic acid. OH H2SO4 2-butanol butan-2-ol 100 °C + H2O 2-butene but-2-ene Elimination Reaction but-1-ene - also produce 1-butene o) Show the reaction between water and cyclohexene. OH H2SO4 + H2O 100 °C Addition Reaction p) Show the oxidation of 2-methylbutan-2-ol O No Reaction