8.1 Practice Worksheet A
advertisement

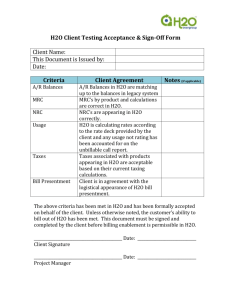
Name: ____________________________________________________ Date: ______________ Period: ____________ 8.1 Practice Worksheet A Balancing Equations Part 1: Why do scientists use chemical equations? Chemical reactions can be represented with both word equations and formula equations. A) Write a word equation for the following reaction: 2 Na + 2 H2O 2 NaOH + H2 Word Equations give us qualitative / quantitative (circle one) information about a reaction. B) Write a formula equation for the following reaction: Aluminum bromide + potassium sulfate Aluminum sulfate + potassium bromide Is the equation you wrote balanced (count up atoms making sure none were lost or gained)? A balanced formula equation, gives us qualitative / quantitative information about a reaction. Part 2: Practice making sure you can count atoms correctly in a compound: 1. KClO3 K: ___ Cl: ___ O: ___ 4. 2 Ca(OH)2 Ca: ___ O: ___ H: ___ 2. Ca(OH)2 Ca: ___ O: ___ H: ___ 5. 3 C6H12O6 C: ____ H: ____ O: ___ 3. (NH4)3PO4 N: ___ H: ___ P: ___ O: ___ To balance a chemical equation, use _____________________ at the beginning of each compound to represent the correct amount of molecules needed conserve all atoms involved in the reaction; this is known as following the ___________________________________________________________ Here are some tips for balancing Start by balancing atoms that only appear once on each side Balance ______________________ ions as units Balance _______ and ______ last Part 3: Practice writing word equations where indicated and balance all equations: pure elements are named as themselves compounds should be named according to ionic, covalent, or acid naming rules 6. ___H2(g) + ___O2(g) ___ H2O(g) Word Equation: ____________________________________________________________________ 7. ___ N2(g) + ___H2(g) ___NH3(g) Word Equation: ____________________________________________________________________ 8. ___S8(g) + ___ O2(g) ___SO3(g) 9. ___N2(g) + ___ O2(g) ___N2O(g) Word Equation: ____________________________________________________________________ 10. ___HgO(s) ___ Hg(l) + ___O2(g) 11. ___CO2(g) + ___ H2O(g) ___ C6H12O6(g) + ___O2(g) 12. ___ Zn(s) + ___ HCl(aq) ___ZnCl2(s) + ___H2(g) Word Equation: ____________________________________________________________________ 13. ___ SiCl4(s) + ___H2O(l) ___ H4SiO4(s) + ___ HCl(aq) 14. ___ Na(s) + ___H2O(l) ___NaOH(aq) + ___H2(g) 15. ___H3PO4(aq) ___H4P2O7(aq) + ___H2O(l) 16. ___C10H16(l) + ___Cl2(g) ___ C(s) + ___ HCl(aq) 17. ___CO2(g) + ___NH3(l) ___OC(NH2)2(l) + ___H2O(g) 18. ___Si2H3(aq) + ___O2(g) ___ SiO2(s) + ___H2O(l) 19. ___Al(OH)3(aq) + ___H2SO4(aq) ___Al2(SO4)3(s) + ___ H2O(l) Word Equation: ____________________________________________________________________ 20. ___Fe(s) + ___O2(g) ___ Fe2O3(s)