Section 16.1-16.3
advertisement

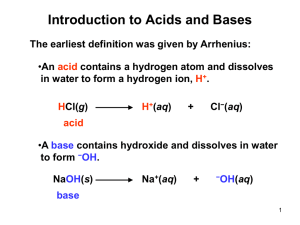
Section 16.1-16.3 1. Define Arrhenius acid/base 2. Define Bronsted-Lowry Acids/bases 3. Define hydronium 4. Draw a proton transfer reaction 5. In Bronsted-Lowry Acids/bases reactions are there always an acid and a base present? 6. Define amphiprotic 7. Define conjugate-base pair? 8. Define Conjugate base 9. Define Conjugate acid 10. (a) What is the conjugate base of HClO4, H2S, PH4+, HCO3 - ? (b) What is the conjugate acid of CN- , SO42- , H2O, HCO3- ? 11. The hydrogen sulfite ion (HSO3-) is amphiprotic. Write an equation for the reaction of HSO3 with water (a) in which the ion acts as an acid and (b) in which the ion acts as a base. In both cases identify the conjugate acid–base pairs. 12. List the three categories of acid/bases on page 677 13. For the following proton-transfer reaction use Figure 16.4 to predict whether the equilibrium lies to the left (Kc < 1) or to the right (Kc > 1): 14. Define Autoionization 15. Define Kw 16. Define ion-product constant 17. Calculate the values of [H+] and [OH-] in a neutral aqueous solution at 25 °C. 18. Calculate the concentration of H+(aq) in (a) a solution in which [OH-] is 0.010 M, (b) a solution in which [OH-] is 1.8 * 10-9M. Note: In this problem and all that follow, we assume, unless stated otherwise, that the temperature is 25 °C. 19. (a) What is the difference between the Arrhenius and the Brønsted–Lowry definitions of an acid? (b) NH3(g) and HCl(g) react to form the ionic solid NH4Cl(s). Which substance is the Brønsted–Lowry acid in this reaction? Which is the Brønsted–Lowry base? 20. (a) Give the conjugate base of the following Brønsted–Lowry acids: (i) HIO3, (ii) NH4+. (b) Give the conjugate acid of the following Brønsted–Lowry bases: (i) O2-, (ii) H2PO4-. 21. Designate the Brønsted–Lowry acid and the Brønsted–Lowry base on the left side of each of the following equations, and also designate the conjugate acid and conjugate base of each on the right side: 22. (a) The hydrogen sulfite ion (HSO3-) is amphiprotic. Write a balanced chemical equation showing how it acts as an acid toward water and another equation showing how it acts as a base toward water. (b) What is the conjugate acid of HSO3-? What is its conjugate base? 23. Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: (a) CH3COO-, (b) HCO3-, (c) O2-, (d) Cl-, (e) NH3. 24. (a) Which of the following is the stronger Brønsted–Lowry acid, HBrO or HBr? (b) Which is the stronger Brønsted–Lowry base, F- or Cl-? 25. Predict the products of the following acid–base reactions, and predict whether the equilibrium lies to the left or to the right of the equation: 26. If a neutral solution of water, with pH = 7.00, is cooled to 10 °C, the pH rises to 7.27. Which of the following three statements is correct for the cooled water: (i) [H+] > [OH-], (ii)[H+] =[OH-], or (iii) [H+] < [OH-]? 27. Calculate [H+] for each of the following solutions, and indicate whether the solution is acidic, basic, or neutral: (a)[OH-] = 0.00045 M; (b) [OH-] = 8.8 * 10-9 M; (c) a solution in which[OH-] is 100 times greater than [H+]. 28. At the freezing point of water (0 °C), Kw = 1.2 * 10-15. Calculate [H+] and [OH-] for a neutral solution at this temperature.