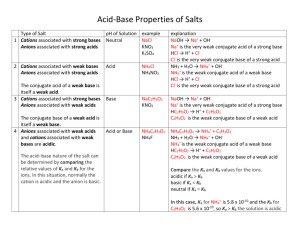
8.6 Acid-Base Properties of Salt Solutions
advertisement
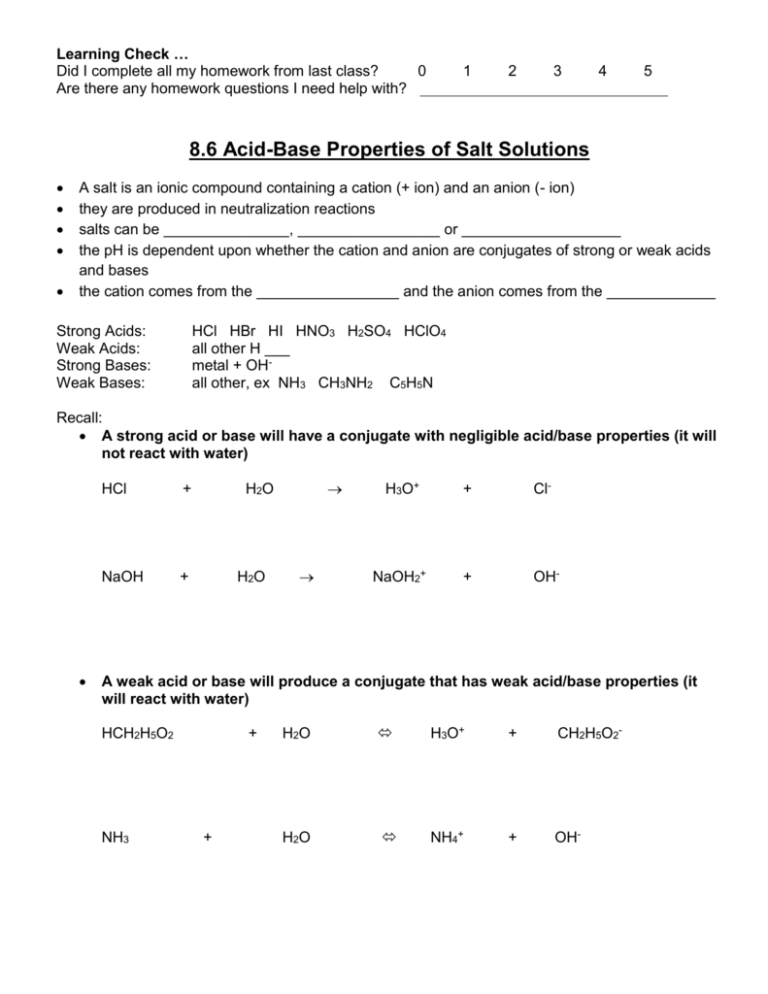
Learning Check … Did I complete all my homework from last class? 0 Are there any homework questions I need help with? 1 2 3 4 5 8.6 Acid-Base Properties of Salt Solutions A salt is an ionic compound containing a cation (+ ion) and an anion (- ion) they are produced in neutralization reactions salts can be _______________, _________________ or ___________________ the pH is dependent upon whether the cation and anion are conjugates of strong or weak acids and bases the cation comes from the _________________ and the anion comes from the _____________ Strong Acids: Weak Acids: Strong Bases: Weak Bases: HCl HBr HI HNO3 H2SO4 HClO4 all other H ___ metal + OHall other, ex NH3 CH3NH2 C5H5N Recall: A strong acid or base will have a conjugate with negligible acid/base properties (it will not react with water) HCl + NaOH + H2O H2O H3O+ + Cl- NaOH2+ + OH- A weak acid or base will produce a conjugate that has weak acid/base properties (it will react with water) HCH2H5O2 NH3 + + H2O H3O+ + CH2H5O2- H2O NH4+ + OH- Salts that Produce Neutral Solutions… Ex: NaCl, Ca(NO3)2, KBr Cation (eg Na+) is the conjugate of a __________ base (eg NaOH) and therefore has ______________ acidic properties. Anion is the conjugate of a ___________ acid (eg HCl) and therefore has ________________ basic properties. Neither of the ions act as an acid or a base and thus the pH is _________________ a salt produced in the reaction of a ______________________ and ___________________ will be _____________________ Salts that Produce Acidic Solutions… Ex: NH4Cl Cation (NH4+) is the __________ conjugate acid of a ___________ base (NH3). This conjugate acid would thus react with water to produce H3O+. NH4+ + H2O NH3 + H3O+ Anion (Cl-) is the conjugate of a ____________ acid (eg HCl) and therefore would have negligible basic properties. Cation acts as an acid; anion has no effect on pH a salt produced in the reaction of a _____________________ and _____________________ will be ______________________ Ex) What is the pH of a 0.35 M NH4Cl solution? (Kb for ammonia is 1.8x10-5) Salts that Produce Basic Solutions… Ex: NaCH3COO • Anion (CH3COO-) is the __________ conjugate base of a _________ acid (CH3COOH). This conjugate base would thus react with water to produce OH-. CH3COO- + H2O CH3COOH + OH- • Cation is the conjugate of a ___________ base (eg NaOH) and therefore will have negligible acidic properties. Anion acts as an base; cation has no effect on pH a salt produced in the reaction of a ______________________ and ____________________ will be _____________________ • • Ex) Calculate the pH of a solution that contains 12.5g of sodium acetate (NaCH3COO) dissolved in 1.0L of H2O. (Ka for acetic acid is 1.8x10-5) What about if the salt contained an ion that is AMPHOTERIC? Ex) Is the solution of KH2PO4 acidic or basic? Ex) Sort the following salts based on whether they form acidic, neutral, or basic solutions when dissolved in water. NaCl NH4NO3 NaClO C5H5NHCl Self Check How prepared am I to start my homework? Can I … … determine the pH of a solution containing a salt LiF 0 1 KCN 2 3