Potassium_Permanganate_Procedure_2015.sflb
advertisement

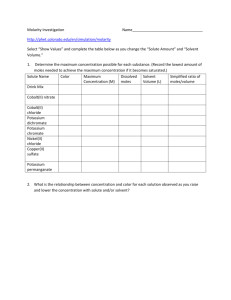
Procedure for the use of Potassium Permanganate soaks in Primary Care Guidance for Community–based Healthcare Professionals Written by Julie Hewish, Senior Tissue Viability Nurse January 2015 The content and structure of this procedure have been adapted with kind permission from Helen Harris, Tissue Viability Nurse, Bath and North East Somerset NHS Trust. Procedure for the use of Potassium Permanganate soaks in Primary Care Page Introduction 3 What is Potassium Permanganate used for? 3 Page 2 of 8 Precautions 3-4 Instructions for use 5-7 Audit and review 7 References 7 Final Version: 15th January 2015: Community Tissue Viability Service Procedure for the use of Potassium Permanganate soaks in Primary Care Potassium permanganate is often prescribed for weeping, exuding skin or for blistered areas that require drying up. It is available as a solution for further dilution and as a tablet preparation, which is dissolved in water and further diluted to a specified concentration. This product is for external use only and can be fatal if ingested orally due to local inflammatory reactions that block the airways or cause perforations of the gastrointestinal tract. This can also lead to death through toxicity and organ failure (NHS England, 2014) (appendix 1) Potassium permanganate must not be used as a first line, stand-alone treatment. Investigations to identify and manage underlying causes of skin deterioration and/or increased exudate levels must be carried out in order to implement a holistic management plan. Potassium permanganate must only be used when all standard treatments have been exhausted. This treatment is usually instigated under the guidance of Dermatology or Tissue Viability. 1.0 BACKGROUND Potassium permanganate is an oxidising agent with disinfectant, deodorising and astringent properties. Its chemical formula is KmnO4. In its raw state potassium permanganate is an odourless dark purple or almost black crystal, granular powder or tablet. The main form of use is a potassium permanganate solution that is made by dissolving crystals or powder in water. Within the Oxford Health NHS Foundation Trust the more convenient tablet form is used to prepare topical solutions. 3.0 PRECAUTIONS Potassium permanganate is dispensed as tablets and concentrated solutions are caustic and can burn the skin. Even fairly dilute solutions can irritate skin and repeated use may cause burns. Staff must therefore ensure that they wear the appropriate protective clothing when working with potassium permanganate (see Section 4). Potassium Page 3 of 8 Final Version: 15th January 2015: Community Tissue Viability Service Procedure for the use of Potassium Permanganate soaks in Primary Care permanganate presents in tablet form which must not be handled as it can stain the skin and turn nails brown but this does resolve. It can be fatal if the tablets or diluted solution is ingested Potassium Permanganate stains floor surfaces, clothing and carpets permanently. Avoid contact with the eyes and buccal mucosa. Do not use soap or detergent in conjunction with treatment as this will react with the solution which will discolour and give off an unpleasant odour. Any leftover tablets should not be disposed of via household waste. Any excess tablets should be returned to a pharmacy for disposal. This may be done by a responsible family member, the patient or the District Nursing Team based upon a robust clinical risk assessment for safe disposal. Do not store any excess diluted solution. The solution should be made up just before it is to be used. If the solution is left, it will start to oxidise and go brown after which time it should not be used. This may also increase the risk of accidental ingestion. Page 4 of 8 Final Version: 15th January 2015: Community Tissue Viability Service Procedure for the use of Potassium Permanganate soaks in Primary Care PROTOCOL FOR USE Potassium Permanganate should be used in practice according to the manufacturer’s instructions and the patient’s prescription. Potassium Permanganate soaks are for external use only and can be fatal if ingested orally due to local inflammatory reactions that block the airways or cause perforations of the gastrointestinal tract. This can also lead to death through toxicity and organ failure (NHS England, 2014) (Appendix 1) Prior to administration: This product should be prescribed for a patient following guidance from a specialist team Treatment should be supported by a clear care plan that reflects the instructions given within this clinical protocol. This should be available within the patients notes. There should be relevant documentation to support medicine administration (e.g Pink Form for District Nurses). This product should be stored and used in line with this clinical protocol and manufacturers recommendations. Both the tablets and solution could be mistaken for sweets or a harmless drink. Children and cognitively impaired individuals are particularly vulnerable. Therefore a documented clinical risk assessment and management plan relating to safe administration and excess tablet disposal should be available in the patient’s notes. Ensure patient informed consent prior to administration. Read the manufacturers guidance on administration and dosage carefully Page 5 of 8 Final Version: 15th January 2015: Community Tissue Viability Service Procedure for the use of Potassium Permanganate soaks in Primary Care Equipment required: Disposable cups, washing up bowl or bucket, bin liner, (preferably white to visualise colour of solution), disposable apron and gloves. • Clean your hands (see standard infection prevention and control policy) • Wear non sterile examination gloves to prevent contact of tablet with skin • In line with manufacturer’s dilution guidance, drop one tablet into a disposable cup and fill with warm tap water to allow the tablet to start dissolving. Caution: Sometimes potassium permanganate tablets are halved for perceived smaller amounts of solution. This must be carried out with extreme caution as the chemical is an irritant to mucus membranes and splitting the tablets will affect the accuracy of the dilution. • At all times ensure the tablet does not drop into the bowl or come into contact with the skin. • Line a washing up bowl or bucket with a bin liner and fill with warm tap water. • When the solution in the cup has gone very dark (the tablet will still be dissolving), carefully add this concentrate to the washing up bowl or bucket. • Do not allow the dissolving tablet to fall into the bowl • The resultant colour in the bowl should be transparent rose pink/purple – NOT DARK PURPLE. • If the resultant solution is too light in colour, add more tap water to the remainder of the tablet in the disposable cup and add to the solution in the washing up bowl. • If the resultant solution is too dark in colour, discard some solution and add more tap water. • Immerse the limb in the solution for 10 minutes. A potassium permanganate solution of 1 in 1000 may be used as wet soaks to blistering wounds or skin. Strips of dressing pack gauze should be soaked in the solution and wrapped around the affected area for 20-30 minutes (DermNet, 2013, accessed 7/1/15) Page 6 of 8 Final Version: 15th January 2015: Community Tissue Viability Service Procedure for the use of Potassium Permanganate soaks in Primary Care • After the treatment, dispose of solution in the toilet. The dilute solution will not stain the toilet as it is a weakened solution and the disposal is transient. After flushing, flush again. • Disposal of the tablet and cup must be in clinical waste. • Always clean hands after removal of protective clothing. (See standard infection prevention and control policy.) Excess Product Disposal Remember this product is for external use only and should be swallowed. Therefore on completion of treatment, excess tablets should not be disposed of via household waste but returned to a pharmacy for disposal. This may be done by a responsible family member or the patient. The District Nursing Team may remove the tablets from the patients home should a clinical risk assessment deem this to be the safest method. Where possible seek consent from the family or patient to remove the prescribed product. 5.0 AUDIT AND REVIEW • In the light of new evidence. • Investigate any adverse events relating to the use of potassium permanganate as per guidance above. Reference: DermNet NZ (2013) Potassium Permanganate. www.dermnetnz.org/treatments/permanganate.html accessed (7/1/2015) NHS England (2014) Patient safety alert: Stage One: Warning Risk of death or serious harm from accidental ingestion of potassium permanganate preparations Page 7 of 8 Final Version: 15th January 2015: Community Tissue Viability Service Procedure for the use of Potassium Permanganate soaks in Primary Care Appendix 1 Page 8 of 8 Final Version: 15th January 2015: Community Tissue Viability Service