Colorimetric determination of manganese in steel
advertisement

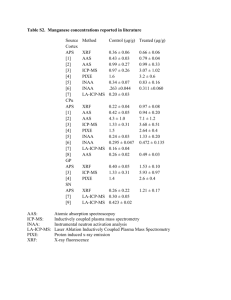
Colorimetric determination of manganese in steel Colorimetric analysis • type of visible spectroscopy • used to determine the concentrations of coloured solutions • coloured substances absorb light of a colour complementary to their own • a single wavelength of light is used • the amount of light absorbed is proportional to the concentration of the substance Colorimetric determination of manganese in steel Manganese in steel can be converted into permanganate ions by oxidising with - nitric acid, Mn(s) Mn2+(aq) + 2e- potassium periodate, Mn2+(aq) + 4H2O(l) MnO4-(aq) + 8H+ + 5ethe colour develops during the second step; the mixture should be heated until the intensity of the pink colour remains constant permanganate solution appears purple because it absorbs green light and transmits red and blue thus a green filter (520 - 550 nm) is used Colorimetric determination of manganese in steel Optically matched cuvettes are used to ensure that any absorption by the cuvette is kept constant A calibration curve is prepared where the absorbance of known concentrations of permanganate is measured