Chapter 5 Metabolism Notes
advertisement

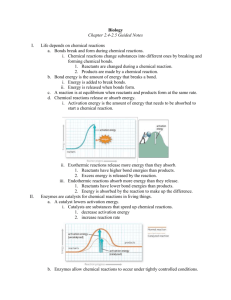
Chapter 5 Metabolism Metabolism is every chemical reaction that occurs with your body, both anabolic and catabolic reactions. Chemical reactions require energy. Energy is defined as the ability to do work. We are familiar with both kinetic and potential energy. All molecules above absolute zero, contain kinetic energy. The bonds which hold molecules together contain potential energy. The 1st law of thermodynamics states that energy cannot be created or destroyed. The 2nd law of thermodynamics states that entropy tends to increase, in other words energy tends to spread spontaneously. Now let’s apply this to some real-life examples: molecules tend to spread from an area of high concentration to an area of low concentration down a concentration gradient. We are familiar with this concept as the property of diffusion. When molecules are close together, they tend to collide readily, as they collide, they ricochet and spread further from one another. Energy spontaneous dispersal is resisted by chemical bonds, chemical bonds are considered a type of potential energy because it can be stored. Energy flow is generally a one-way flow. Worked occurs as a result of energy transfers. The conversion of light energy into chemical energy, during the process of photosynthesis is one example. Living things constantly use energy to grow, to move, to acquire nutrients, to reproduce, and so on. Some energy is lost in every one of these processes, and unless the energy is replenished life will end. Most of the energy fuels life on earth comes from the sun. Each energy transfer from producer to primary consumer, to secondary consumer, and so on. Only transfers from 5 to 30% of the energy directly to the organism for growth. The rest is lost, and as heat. It’s time for chemistry review! Reactants→ products. If reactants have less free energy, then the products the reaction will not proceed without energy input. We say that such reactions are endergonic. If the reactants have more free energy than the products the reaction will release free energy. Such reactions are exergonic. Activation energy is the minimum amount of energy required to get a chemical reaction started. Some chemical reactions require so much energy that they would never proceed if it weren’t for enzymes. A crazy example is a substance called guncotton. Guncotton had such a low activation energy that many of the factories which try to produce it actually burned down. It was a form of nitrocellulose. It was supposed to be marketed as a weapon, but gunpowder was much safer to use. ENZYMES!! Within cells the making and breaking of chemical bonds and most often requires enzymes. In a process called catalysis, and enzyme makes a reaction run much faster than what on its own. Enzymes are unchanged by participating in a reaction so they can work again and again. Some enzymes are RNAs, but most are proteins their shape is highly specific and each kind recognizes a specific reactant or substrate and alters them in a specific way. Each enzyme is a polypeptide chain which folds into one or more active sites, which are pockets where substrates bind and where reactions proceed. Enzyme’s lower the activation energy of many chemical reactions, speeding them up by tens of thousands to hundreds of thousands times faster than they would go on their own. Enzymes allow chemicals to reach the transition state. The transition state is when substrates bonds reach their breaking point and the reaction will run spontaneously to products. The enzymes have a few specific ways that they usually help out… Binding at an active site often brings 2 or more substrates close together, allowing for better contact. Interacting with a substrate molecule causes the enzyme to change shape so that the fit between them improves, this is called the induced fit model. Orienting substrates in positions that favor reactions simply means they align the substrates appropriately. The active sites of some enzymes repel water, shutting out water molecules so they don’t interfere. A few factors that affect the efficiency of enzyme activity are pH, temperature, and salinity. Adding energy in the form of heat boosts free energy, molecular motion increases and brings the reactants closer to their activation energy. But increasing thermal energy only works to a certain point because enzymes to nature above, a characteristic temperature, making them ineffective. PH tolerance of enzymes varies depending on their job. Optimal pH between 6 and 8 is most common, however the enzyme pepsin, which works in your stomach requires a pH of between one and 2. Salinity also affects enzyme activity by affecting their tertiary structure (shape) too little salt in the surrounding fluid effects. The functional groups and their ionic attraction towards one another too much salt interferes with hydrogen bonding. Either way, the shape of the enzyme changes, and is said to be denatured. Metabolic pathways or biochemical pathways are when building, rearranging or breaking down of organic substances occurs step by step. Some are linear, but many are cyclical. Several of these pathways would release too much energy if a molecule were broken down all at once. So the energy is released in small amounts step-by-step. Cells must adjust to the types and amounts of molecules that produces many reactions do not only run from reactants to products, but the other way around depending on the concentration of relative products to reactants. FOR EXAMPLE – the end product of a series of in enzymatic reactions may inhibit the activity of one of the enzymes in the series an effect called feedback inhibition. Some regulatory molecules inhibit or activate and enzyme by binding directly to its active site, others bind to allosteric sites. Allosteric sites are regions other than the active site where regulatory molecules may bind enhancing or inhibiting its function. Do you remember redox reactions??? Redox reactions form ionic bonds. One molecule accepts electrons is said to be reduced and becomes an anion. The next molecule gives away electrons is said to be oxidized and becomes a cation. Redox reactions are often called electron transfers. An electron transfer chain is an organized series of reaction steps in which membrane-bound arrays of enzymes and other molecules give up and accept electrons in turn. Electrons are often at a higher energy level when they enter a chain than when they leave. This is also called an electron transport chain or E TC, such as the one found in the process of the light reaction in photosynthesis. Most enzymes don’t function properly without assistance from metal ions or small organic molecules. These helpers are called co-enzymes or cofactors. Cofactors are often dietary vitamins and minerals of a metallic form which can act easily as an electron donator or acceptor to help bring on the transition state of a reactant. So enzymes are organic molecules which carry chemical groups, atoms or electrons from one reaction to another in metabolic pathway. NADP+ which is an electron acceptor in the light reaction forms NADP H after also receiving hydrogen ions and then carries energy to the Calvin cycle. ATP is considered a special coenzyme as phosphate groups are transferred to and from reactions, allowing energy to be transferred. Phosphorylation is when a phosphate group is transferred from one molecule to another. The nucleotide coenzyme ATP has 3 phosphate groups, and the bonds between these groups hold a lot of energy. They are often called high energy phosphate bonds. Cells cannot store ATP, but can store ADP readily. The ATP/ATP cycle couples endergonic reactions with exergonic ones. This cycle fuels many cell processes.