P262 DALTEPARIN VENOUS THROMBO EMBOLISM
advertisement

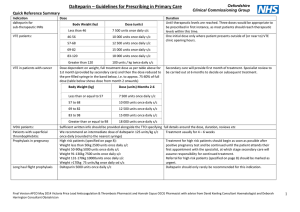
P262 DALTEPARIN VENOUS THROMBO EMBOLISM PROPHYLAXIS IN PATIENTS WITH RENAL IMPAIRMENT MN Ali1, KL Allan1, SW Lines1, S Ackroyd 2, J Stoves 1 1. Department of Renal Medicine, Bradford Royal Infirmary (BRI) 2. Department of Haematology, Bradford Royal Infirmary (BRI) Introduction: According to House of Commons Committee report in 2005; 25,000 patients in the UK die from preventable hospital-acquired venous thrombo-embolism (VTE). The momentum to reduce harm and deaths associated with VTE increased in 2010 by linking performance measures with the financial status of NHS organisations in England. Monitoring structures have been put in place to ensure that VTE risk assessments are completed at the time of hospital admission. Various forms of LMWH are used for prophylactic anti-coagulation of hospitalised patients. Other forms of prophylaxis include Thrombo-Embolic Deterrent (TED) stockings, oral Rivaroxaban and intermittent pneumatic compression. Dalterparin at a dose of 5,000 units is used for routine prophylaxis for patients with medical conditions. Reduced dose Dalteparin has been used in patients with severe renal failure or in dialysis dependent patients; however there is insufficient data on the use of the full dose in this cohort of patients. We conducted an audit to establish the safety of standard dosing of Dalteparin prophylaxis in patients with renal impairment. Method: This audit took place at between November 2012 to February 2013. The Inclusion criteria were: age > 18 and single agent prophylactic anticoagulation with s/c Dalteparin for six days or more. Factor Xa levels were measured at 2-3 hours post dose. The following data were recorded: age, sex, weight, dialysis dependence, serum Creatinine (Cr), estimated Glomerular filtration rate (GFR), serum albumin, Dalteparin dose, number of doses administered and number of missed doses, additional anticoagulation administered during dialysis, and the timing of factor Xa level sampling in relation to the previous dose of Dalteparin. There were 28 patients in total,15 male and 13 female with age varying from 40’s to 80’s. There were 15 patients on Haemodialysis, 10 had AKI, 4 had CKD, 4 acute on chronic renal impairment and 2 general medical patients with GFR >90. GFR ranged from 8147mls/min, with mean 21.8mls/min (+- SD). Results: 30 levels were taken in patients receiving doses of 5000 units of Dalteparin and, 5 levels in patients receiving 2500 units of Dalteparin. There was no significant correlation between factor Xa levels and measures of renal function in non-dialysed patients, and no significant difference in factor Xa levels between patients according to prescribed dose of Dalteparin. There was however a significant correlation between factor Xa levels and body weight with increase in factor Xa levels in patients with low body weight which needs to be investigated further. Conclusion: Drug accumulation is thought to be less problematic with Dalteparin and Tinzaparin than Enoxaparin. In this study, standard dose prophylactic Dalteparin was not associated with a significant increase in factor Xa activity in patients with increasingly severe renal impairment, nor in the dialysis dependent population. Significant accumulation did not occur in the 15 haemodialysis patients receiving standard dose prophylaxis plus additional doses of LMWH on dialysis sessions. Importantly, there were no identified bleeding events, nor any incidence of VTE. Dalteparin 5000 OD should be used for VTE prophylaxis in medical patients with all degrees of chronic kidney disease and for dialysis dependent patients. Dose reduction is not routinely required. Factor Xa monitoring is advisable in renal patients with additional risk factors including: specific additional bleeding risk, Deranged LFT’s, Low BMI, Prolonged use of the drug >10 doses. Therapeutic LMWH dosing in renal patients is not addressed in this study.