(Nabertherm GmbH), with a continuous flow of nitrogen (100 cm 3
advertisement

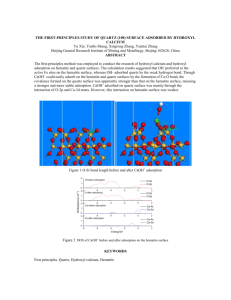
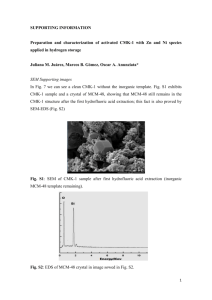
Carbon Dioxide Sequestration and Methane Removal from Exhaust Gases Using Resorcinol-Formaldehyde Activated Carbon Xerogel Ahmed Awadallah-F1 and Shaheen A. Al-Muhtaseb*1 Department of Chemical Engineering, Qatar University, P.O. Box 2713, Doha, Qatar Online Resource Sections Contents S1. Carbonization and activation of xerogel ........................................................ 2 S2. Characterization ............................................................................................ 2 S3. Adsorption measurements ......................................................................... 3-6 S4. References .................................................................................................... 7 * Corresponding Author. Tel.(+974) 4403-4139; Fax: (+974) 4403-4131; E-mail: s.almuhtaseb@qu.edu.qa 1 On leave from the National Center for Radiation Research and Technology, P.O. Box 29, Nasr City, Cairo, Egypt 1 S1. Carbonization and activation of xerogel The dried RF xerogel was placed in a ceramic boat inside a programmable electric-heated tube furnace (Nabertherm GmbH), with a continuous flow of nitrogen (100 cm3/min). The furnace was first maintained at room temperature for 30 min to make sure that the air is completely purged with the flowing nitrogen. Then, the furnace was heated up to a temperature of (773 K) with a heating rate of 10 K/min. The sample was maintained at 773 K for 3 h, and then allowed to cool to room temperature while passing nitrogen. The resulting carbon xerogel was then activated in the same tube furnace with CO2 flow (150 cm3/min) instead of nitrogen, heating the sample again with a rate of 10 K/min to 973 K, maintaining this temperature for 1 h, and then allowing the sample to cool down to room temperature while passing CO2. S2. Characterization The pore structure of the RF activated carbon xerogel (RF-ACX) was measured using the adsorption of nitrogen at 77 K by a Micromeritics ASAP2420® Accelerated Surface Area and Porosimetry System with an enhanced micropore capability (utilizing 1-Torr pressure transducer) as summarized in Table 1 and Fig. 1. Prior to the adsorption measurements, the samples were regenerated in-situ for 8h at 423 K under vacuum (1×10-4 Pa). The total pore volume was calculated from the adsorbed volume of nitrogen at the temperature of 77 K and the relative pressure (P/P0) of 0.99 (99% of the saturation pressure). The pore size distributions and surface energy distribution were obtained by density functional theory (DFT) and modified density functional theory calculations, respectively. The morphology of the RF-ACX was observed with a scanning electron microscope (SEM, Philips 515) as summarized in Fig. 2. 2 S3. Adsorption measurements Adsorption measurements of pure CO2, CH4 and N2 onto RF-ACX were performed using a HyGra™ Microbalance with the Microgram option (Rubotherm, Germany). This equipment depends on a magnetic suspension balance (MSB) which overcomes the disadvantages of other gravimetric instruments by separating the sensitive microbalance from the sample and the measuring atmosphere (Dreisbach et al. 2003; Hill 1949). This system is able to perform adsorption measurements in a wide range of gas pressures up to ~25 MPa. The adsorption temperature may also be controlled within the range of 293–423 K. In a typical adsorption experiment, the adsorbent is weighed and placed in a basket (sample holder) suspended through an electromagnet. The cell in which the basket is housed is then closed and vacuum or high pressure is applied. Before the first run, an empty sample holder is placed in the cell and the system is evacuated to 5 kPa. Then, helium is dosed gradually into the system at a fixed temperature, while monitoring the variation of the weight of the sample holder as a function of pressure. When the helium gas pressure increases, its density will also increase. This leads to a buoyancy (flotation) effect which reduces the measured (apparent) weight of the sample holder according to Archimedes theorem. The mass and volume of the empty sample holder (Mh and Vh, respectively) can be estimated by analyzing the apparent (false) weight reduction of the sample holder with increasing pressure as a result of buoyancy. Whenever a new adsorbent sample is placed into the system, it is first regenerated in-situ at 373 K under a vacuum of 5 kPa for 24 h. Then, a first pressurization experiment is performed by gradual dosing of helium at 333 K and monitoring the variation of the sample’s apparent weight as a function of pressure. Assuming that helium adsorption at this condition is virtually 3 nil, the actual mass of the adsorbent shall remain constant regardless of the system pressure. As a result, the measured apparent weight reduction with increasing pressure is again attributed solely to buoyancy effects. The volume and mass of the adsorbent sample (Vs and Ms, respectively) are estimated through analyzing such buoyancy effects on the apparent weight. The gravimetric method is an accurate technique to measure the adsorption equilibrium of pure gases. However, when an adsorbent is immersed within a fluid of a certain density, the effect of buoyancy force should be considered as a correction to adjust the measured (apparent) weight of the sample. The measured apparent weight corresponds to what is known as the “excess amount adsorbed” (nex), which is obtained directly from experimental measurements as nex M M s M h Ms . Mw (1) where M is the measured total weight at the system pressure and temperature, and Mw is the molecular mass of the adsorbate. The actual amount adsorbed (denoted as “absolute quantity adsorbed”, nads) corresponds to actual weight of the sample with the proper buoyancy correction. Employing this correction according to Archimedes theorem, the absolute amount adsorbed from experimental data is (Cavenati et al. 2004): V V s nads nex g h Ms where ρg is the molar density of the gas. 4 (2) Before conducting the first adsorption experiment with the required adsorbate, the adsorbent sample is regenerated in-situ at 373 K under a vacuum of 5 kPa for 24 h. Then, the system temperature is set at the highest measured temperature (333 K), and the test gas is dosed gradually with pressure increments of ~ 100 kPa, while allowing 1 h of equilibration time for each pressure increment before recording the apparent weight corresponding to each pressure segment. Visual display confirms the stabilization of the measured weight (indicating equilibrium) at each pressure segment before going to the next one. Gas dosing continues up to a maximum pressure of ~ 1 MPa. Afterwards, the gas is discharged gradually (with decreasing pressure increments of ~ 100 kPa), while allowing similar equilibration times for each increment, until reaching a vacuum of 5 kPa. This constitutes a complete adsorption/desorption experiment at the constant temperature of 333 K. The previous procedure (in-situ regeneration, adsorption and desorption) is repeated successively at the required temperatures in a decreasing manner (i.e., starting by 333 K, followed by 323 K, then 313 K, then 303 K, and finally 293 K). The measured (excess) adsorption/desorption are summarized in Figure S1, along with their buoyancy-corrected (absolute) equivalents. 5 Fig. S1. Measured excess (solid symbols) and absolute (empty symbols) adsorption/desorption isotherms for: (a) CO2, (b) CH4 and (c) N2 onto RF-ACX at different temperatures 6 S4. References Dreisbach, F., Seif, R., Losch H.W.: Adsorption equilibria of CO/H2 with a magnetic suspension balance. Purely gravimetric measurements. J. Therm. Anal. Calorim. 71, 73-82 (2003) Hill, T.L., Statistical mechanics of adsorption. adsorption. J. Chem. Phys. 17, 520-535 (1949) V. thermodynamics and heat of Cavenati, S., Grande, C.A., Rodrigues, A.E.: Adsorption equilibrium of methane, carbon dioxide, and nitrogen on zeolite 13X at high pressures. J. Chem. Eng. Data 49, 1095-1101 (2004) 7