Slide 1 - e-CTLT
advertisement

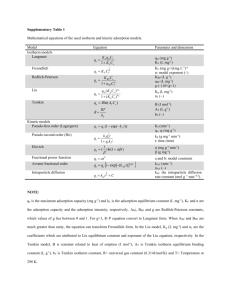
Adsorption:- The formation of a layer of gas on the surface of solid eg.gas like O₂,H₂,NH₃ get adsorbed on the surface of charcoal. Adsorbate:- The substance adsorbed is known as adsorbate. Adsorbent:-The substance on which adsorption takes place is known as adsorbent. There are two types of adsorption:- Physisorption:-Adsorbate is held to the adsorbent by weak Van der waal’s forces. Chemisorption:-adsorbate is held to the adsorbent by strong chemical bond. Adsorption Isotherm:- The relation between extent of adsorption (x/m) and pressure of a gas at constant temperature is known as adsorption isotherm. When extent of adsorption x/m(x is the amount of adsorbate , m is mass of adsorbent is plotted against pressure at constant tempt.). Curve thus obtained is known as adsorption isotherm.. An adsorption isotherm. The isotherm show that at the start, there is linear increase in the extent of adsorption as the pressure is increase .so x/m is proportional to p. At very high pressure ,the saturation point is reached and x/m does not change much with increase of pressure . However for moderate range of pressure the variation of x/m with p is intermediate of two extreme case . Freundlich adsorption isotherm:- Freundlich in 1909 gave an empirical relationship between the quantity of a gas adsorbed by unit mass of solid adsorbent and pressure at particular tempt. The relationship can be expressed by x/m=k.p1/n(n>1) where n=mass of the adsorbed . m=mass of adsorbent at pressure p k&1/n=constant which depend on the nature of the adsorbent (Evaluated experimentally) The value if 1/n is between 0 and 1.The variation x/m with pressure is shown in figure.The curve is also known as Freundlich isotherm. •When 1/n=1, the friundlich equation explain the first part of the isotherm x/m=kp for x/m proportional to p(adsorption varies directly at p) * when 1/n=0,the equation explain the last part of the isotherm x/m=k.p0 =k i.e;saturation point reached when x/m becomes maximum after which there is no change in x/m at pressure is further increased (so that adsorption is independent of p) • When 1/n is between 0to1, the equation accounts for the middle portion of the isotherm. • Evaluation of k and 1/n of a Freundlich Isotherm:- On taking logarithm of Freundlich equation we get logx/m=1/nlogp+logk.This is similar to an equation of straight line y=c+mx. Thus a plot of log x/m v/s logp hives a straight line shown in the figure from the plot we find that : Intercept =logk Slope =1/n “ “Freundlich Isotherm fails in case of high concentration of adsorbate. It is also fails at high pressure.” Application of adsorption:Washing of cloths – The cleaning action of shoaps and detergent is governed by adsorption of grease and oil by hydrocarbon. froth floatation process:- In the froth floatation process the desire sulphide ore is separated from their gangue. The metal sulphide is adsorbed on the surface. Silica gel and Alumina gel are used to adsorbed water vapour and control humidity. Evaluation:1. What is adsorption isotherm? 2. Describe Freundlich Isotherm. 3. Discuss the effect of pressure and temperature on the adsorption of gases on solid. 4. Write a mathematical expression showing relationship between amount of solute adsorbed pr unit mass of the solid adsorbent and concentration of the solute in the solution.