word - Seattle Central College
advertisement

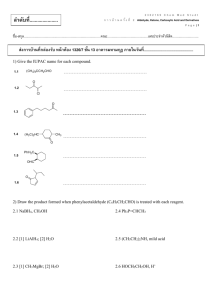
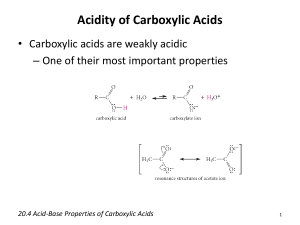
CHEM 122: Introduction to Organic Chemistry Chapter 10: Carboxylic Acids. 1. Name and draw structural formulas for the four carboxylic acids with the molecular formula C5H10O2. Which of these carboxylic acids are chiral? 2. Write the IUPAC name for each carboxylic acid. COOH OH HOOC a) COOH b) OH c) CCl3COOH 3. Draw a structural formula for each carboxylic acid. a) b) c) d) 2-Aminopropanoic acid 3,5-Dinitrobenzoic acid Dichloroacetic acid o-Aminobenzoic acid 4. Draw a structural formula for each salt. a) b) c) d) e) f) Sodium benzoate Lithium acetate Ammonium acetate Disodium adipate Sodium salicylate Calcium butanoate 5. Propanedioic (malonic) acid forms an intramolecular hydrogen bond in which the H of one COOH group forms a hydrogen bond with an O of the other COOH group. Draw a structural formula to show this internal hydrogen bonding. (There are two possible answers.) 6. The following compounds have approximately the same molecular weight: propanoic acid, 1-butanol, and diethyl ether. Arrange them in order of increasing boiling point. 7. Arrange these compounds in order of increasing solubility in water: acetic acid, pentanoic acid, decanoic acid. 8. Complete the equations for these acid-base reactions. OH + NaOH CH3 a) COO-Na + + HCl OH b) COOH + H2NCH2CH2OH OCH3 c) COOH + NaHCO3 d) 9. Formic acid is one of the components responsible for the stinging of biting ants and is injected under the skin by bee and wasp stings. The pain can be relieved by rubbing the area of the sting with a paste of baking soda (NaHCO3) and water, which neutralizes the acid. Write an equation for this reaction. 10. The normal pH range for blood plasma is 7.35 to 7.45. Under these conditions, would you expect the carboxyl group of lactic acid (pKa 4.07) to exist primarily as a carboxyl group or as a carboxylate anion? Explain. 11. The pKa of ascorbic acid is 4.10. Would you expect ascorbic acid dissolved in blood plasma, pH 7.35-7.45, to exist primarily as ascorbic acid or as ascorbate anion? Explain. 12. Complete the equations for the following acid-base reactions. Assume one mole of HCl per mole of amino acid. CH3CHCOO-Na+ a) + HCl(aq) + HCl(aq) NH2 CH3CHCOO-Na+ b) NH3 + 13. Complete these examples of Fischer esterification. In each case, assume an excess of the alcohol. H+ CH3COOH + HO CH3COOH + HO a) H+ b) COOH + CH3CH2OH H+ COOH c) 14. From what carboxylic acid and alcohol is each ester derived? O O O O O a) O b) O O c) CH3OCCH2CH2COCH3 d) 15. Methyl 2-hydroxybenzoate (methyl salicylate) has the odor of wintergreen. This compound is prepared by Fischer esterification of 2-hydroxybenzoic acid (salicylic acid) with methanol. Draw a structural formula for methyl 2hydroxybenzoate. 16. Show how you could convert cinnamic acid to each compound. O OH O OH OH O trans-3-Phenyl-2-propenoic acid (Cinnamic acid) OCH 2 CH 3 17. Give the expected organic product formed with phenylacetic acid, C6H5CH2COOH, is treated with each of the following reagents: a) b) c) d) e) f) g) NaHCO3, H2O NaOH, H2O NH3, H2O LiAlH4 then H2O NaBH4 then H2O CH3OH + H2SO4 (catalyst) H2/Ni 18. Procaine (its hydrochloride salt is marketed as Novocaine) was one of the first local anesthetics developed for infiltration and regional anesthesia. It is synthesized by the following Fischer esterification: O OH + N HO Fischer esterification Procaine H2N 4-Aminobenzoic acid a) b) c) d) 2-Diethylaminoethanol Draw the structural formula of Procaine. Which of the two nitrogen atoms in Procaine is the stronger base? Draw the structural formula of Novocaine (the hycrochloride salt of Procaine). Would you predict Procaine or Novocaine to be more soluble in blood? 19. Methylparaben and propylparaben are used as preservatives in foods, beverages, and cosmetics. Show how each of these preservatives can be prepared from 4aminobenzoic acid. O OCH 3 H2N Methyl-4-Aminobenzoate (Methylparaben) O OH O H2N O 4-Aminobenzoic acid H2N Propyl-4-Aminobenzoate (Propylparaben) 20. When 5-hydroxypentanoic acid is treated with an acid catalyst, it forms a lactone (a cyclic ester). Draw a structural formula for this lactone.