20.4 Acid-Base Properties of Carboxylic Acids
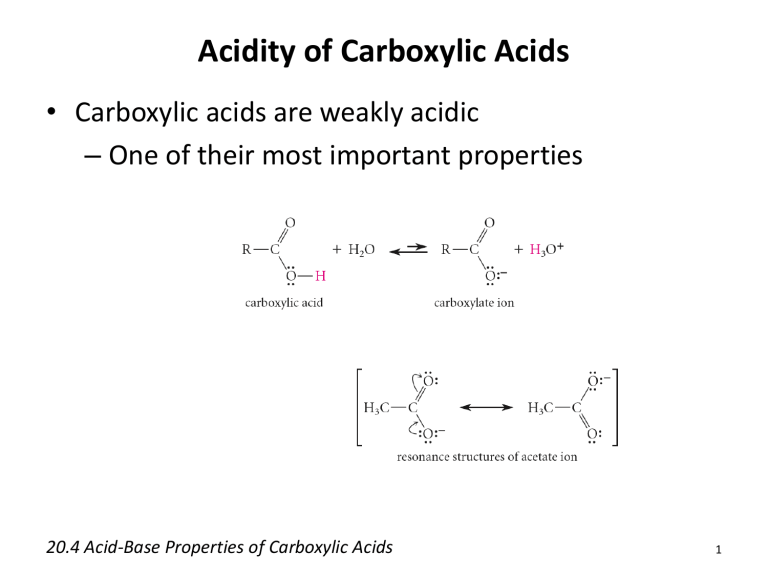
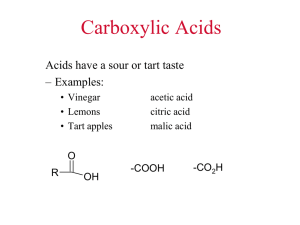
Acidity of Carboxylic Acids
• Carboxylic acids are weakly acidic
– One of their most important properties
20.4 Acid-Base Properties of Carboxylic Acids
1
2
3
pK a
Values of Some Carboxylic Acids
20.4 Acid-Base Properties of Carboxylic Acids
4
Effects of Substitution on Acidity
5
• Conjugate bases of carboxylic acids are called carboxylate ions
– More soluble than carboxylic acids
• Naming carboxylic acid salts
– Name the cation
– Name the anion by removing the “-ic acid” and replacing it with “-ate”
6
Basicity of Carboxylic Acids
• The carbonyl oxygen is weakly basic
– plays an important role in many reactions
• Note: Carboxylate protonation is less favorable
20.4 Acid-Base Properties of Carboxylic Acids
7
Problems
1) You have a mixture of naphthalene and benzoic acid that you wish to separate. How might you take advantage of the acidity of one component in the mixture to accomplish the separation?
2) Without looking at a table of pKa values, rank the substances in each of the following groups in order of increasing acidity: fluoroacetic acid, 3-fluoropropanoic acid, iodoacetic acid
8
Synthesis of Carboxylic Acids
1) Oxidation of primary alcohols
2) Side-chain oxidation of alkylbenzenes
9
3) Grignard or organolithium with carbon dioxide
10
Problem
• How would you prepare butanoic acid using two different methods?
11
Carboxylic Acid Reactions
• There are four main types of reactions:
1. Reactions at the carbonyl group
2. Reactions at the carboxylate oxygen
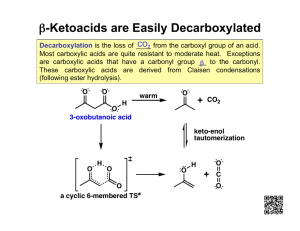
3. Loss of the carboxy group as CO
2
(decarboxylation)
4. Reactions involving the a
-carbon (Ch 22)
20.7 Introduction to Carboxylic Acids Reactions
12
Acid-Catalyzed Esterification
• Ester:
• Carboxylic acid + alcohol + acid catalyst = Ester
• Also referred to as the Fischer esterification
• Equilibrium is favorable, but not large
• The alcohol needs to be present in large excess
(commonly as the solvent)
13
14
15
Problem
• Write out the complete mechanism for the following reaction
16
Esterification via S
N
2 Rxn with Carboxylate Anion
17
Esterification by Alkylation
• The carboxylate oxygen acts as a nucleophile
20.8 Conversion of Carboxylic Acids into Esters
18
Problems
1) What reactants can you use to form the following ester using an S
N
2 reaction?
2) Draw the mechanism for the esterification of benzoic acid via alkylation using diazomethane.
19