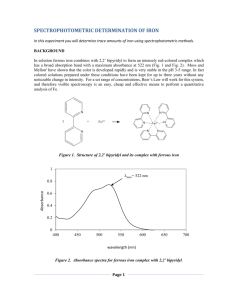
Hmwk #10
advertisement
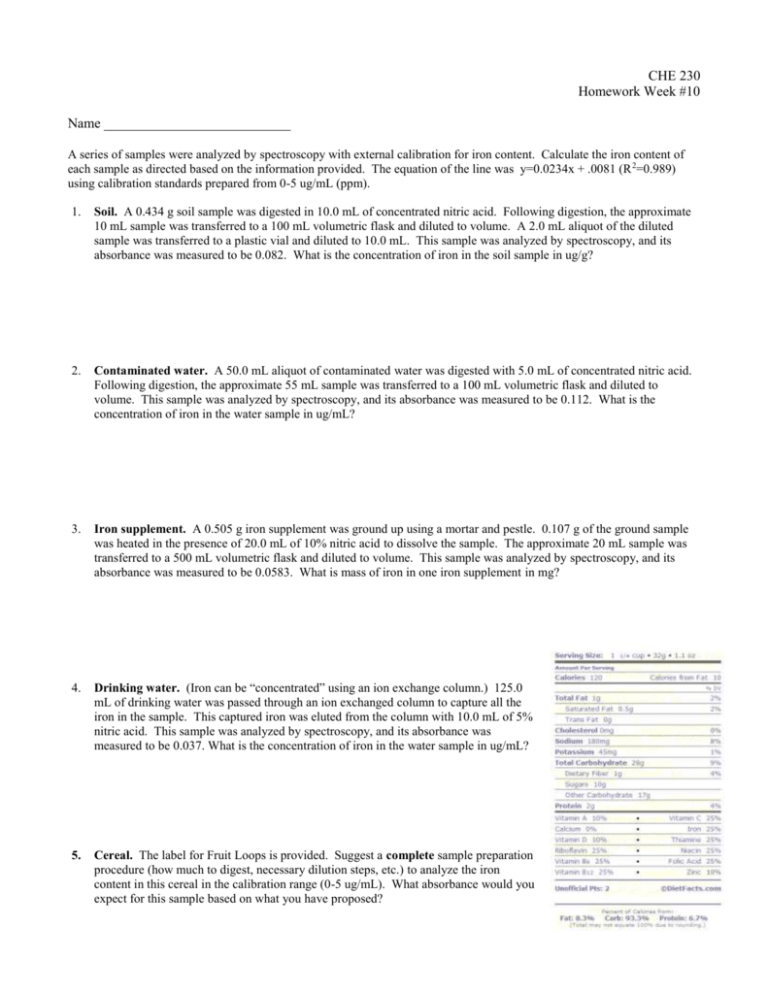
CHE 230 Homework Week #10 Name ___________________________ A series of samples were analyzed by spectroscopy with external calibration for iron content. Calculate the iron content of each sample as directed based on the information provided. The equation of the line was y=0.0234x + .0081 (R 2=0.989) using calibration standards prepared from 0-5 ug/mL (ppm). 1. Soil. A 0.434 g soil sample was digested in 10.0 mL of concentrated nitric acid. Following digestion, the approximate 10 mL sample was transferred to a 100 mL volumetric flask and diluted to volume. A 2.0 mL aliquot of the diluted sample was transferred to a plastic vial and diluted to 10.0 mL. This sample was analyzed by spectroscopy, and its absorbance was measured to be 0.082. What is the concentration of iron in the soil sample in ug/g? 2. Contaminated water. A 50.0 mL aliquot of contaminated water was digested with 5.0 mL of concentrated nitric acid. Following digestion, the approximate 55 mL sample was transferred to a 100 mL volumetric flask and diluted to volume. This sample was analyzed by spectroscopy, and its absorbance was measured to be 0.112. What is the concentration of iron in the water sample in ug/mL? 3. Iron supplement. A 0.505 g iron supplement was ground up using a mortar and pestle. 0.107 g of the ground sample was heated in the presence of 20.0 mL of 10% nitric acid to dissolve the sample. The approximate 20 mL sample was transferred to a 500 mL volumetric flask and diluted to volume. This sample was analyzed by spectroscopy, and its absorbance was measured to be 0.0583. What is mass of iron in one iron supplement in mg? 4. Drinking water. (Iron can be “concentrated” using an ion exchange column.) 125.0 mL of drinking water was passed through an ion exchanged column to capture all the iron in the sample. This captured iron was eluted from the column with 10.0 mL of 5% nitric acid. This sample was analyzed by spectroscopy, and its absorbance was measured to be 0.037. What is the concentration of iron in the water sample in ug/mL? 5. Cereal. The label for Fruit Loops is provided. Suggest a complete sample preparation procedure (how much to digest, necessary dilution steps, etc.) to analyze the iron content in this cereal in the calibration range (0-5 ug/mL). What absorbance would you expect for this sample based on what you have proposed?