Unit 1 lesson 2 What are the Properties of Matter? can have many
advertisement
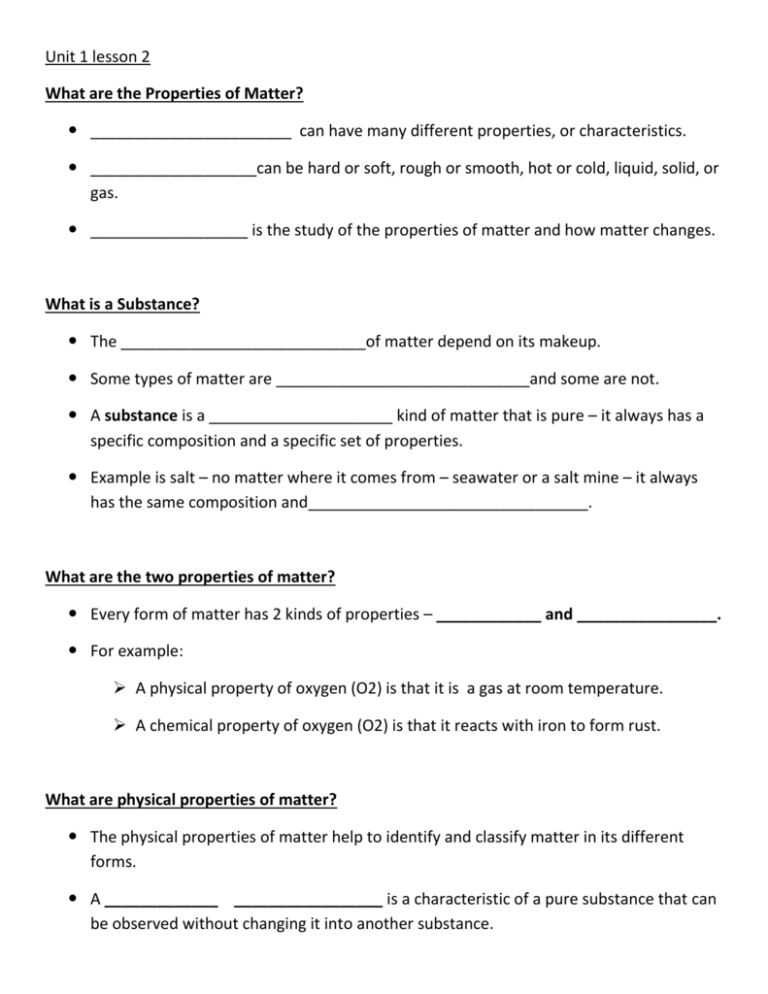
Unit 1 lesson 2 What are the Properties of Matter? _______________________ can have many different properties, or characteristics. ___________________can be hard or soft, rough or smooth, hot or cold, liquid, solid, or gas. __________________ is the study of the properties of matter and how matter changes. What is a Substance? The ____________________________of matter depend on its makeup. Some types of matter are _____________________________and some are not. A substance is a _____________________ kind of matter that is pure – it always has a specific composition and a specific set of properties. Example is salt – no matter where it comes from – seawater or a salt mine – it always has the same composition and________________________________. What are the two properties of matter? Every form of matter has 2 kinds of properties – ____________ and ________________. For example: A physical property of oxygen (O2) is that it is a gas at room temperature. A chemical property of oxygen (O2) is that it reacts with iron to form rust. What are physical properties of matter? The physical properties of matter help to identify and classify matter in its different forms. A _____________ _________________ is a characteristic of a pure substance that can be observed without changing it into another substance. All of our 5 ________________________are used to identify physical properties. What are physical properties of matter? • __________________and______________________________ are physical properties. • Changing the mass or volume of a substance does not change the substance’s _________________________. • The _________ of ____________________is a physical property. The state of matter is the _______________________form of the matter. • Most ___________________________ exists as a solid, liquid, gas, or plasma. What are physical properties of matter? • _____________________conductivity is a measure of how well electric currents move through a substance. • ________________________is the measure of the amount of matter in a given volume. • _______________________conductivity is the rate at which a substance transfers heat. What are Physical Properties of Matter? ______________________is the ability of a substance to dissolve in another substance. Whether or not a substance dissolves in water is also a _________________property. For example, sugar will dissolve in water, but iron will not. What are Physical Properties of matter? ___________________________is the ability of a substance to be rolled or pounded into various shapes. Magnetic attraction is also a _______________________property that can be observed. Some metals, such as iron, are magnetized – they can be attracted to a magnet. What are physical properties of matter? • The shine, or ______________________ of a metal can be easily observed. • The _____________________point of a substance is the temperature at which it changes from a solid to a liquid. • The_____________________ point of a substance is the point at which the substance boils (liquid to gas). What are Chemical Properties of Matter? Unlike physical properties of matter, some properties can’t be observed just by looking at or touching a substance. • A__________________________ property describes the ability of a substance to change into a new substance with different properties. • The ability to _________________or tarnish is a chemical property. When a metal rusts or tarnishes, it changes to a different substance. What are Chemical Properties of Matter? One _______________________property of iron is that it will combine slowly with oxygen in the air to from a different substance – rust. Silver will react with sulfur in the air to form tarnish. In contrast a ____________________property of gold is that it does not react easily with oxygen or sulfur. What are chemical properties of matter? • Chemical properties can be identified by the ___________________ they produce. • __________________is the ability of a substance to burn. Wood has the ability to burn. ________________________is the ability of a substance to interact with another substance and form one or more new substances. Vinegar and baking soda react to make carbon dioxide and water What is the difference between physical and chemical properties? • Physical properties can be observed ____________changing the identity of a substance. • Chemical properties can be observed only by _____________the identity of a substance. At the Scene • The collection and study of physical evidence in a criminal investigation is known as ____________________science. • Ashes from an arson scene can be heated to determine chemicals used to start a fire. • Flecks of paint and the analysis of fibers can provide clues to criminal cases. How can physical and chemical properties identify a substance? • Properties unique to a substance are its_____________________________ properties. • Characteristic properties stay the ______________ regardless of the amount of the sample. They can help identify a substance. • Characteristic properties can be __________________ properties such as density, or ________________________properties, such as flammability.