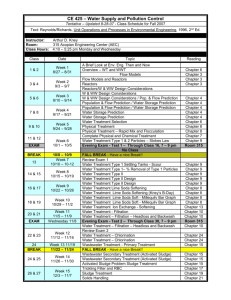
ThesisSaraBrallier - University of Washington
advertisement

Liming effects on trace metal plant availability of a coarse-textured sewage sludge soil. by Sara Brallier a thesis submitted in partial fulfillment of the requirements for the degree of Masters of Science University of Washington 1992 Approved by (Chairperson of Supervisory Committee) Program Authorized to Offer Degree Date Master's Thesis In presenting this thesis in partial fulfillment of the requirements for a Master's degree at the University of Washington, I agree that the library shall make its copies freely available for inspection. I further agree that extensive copying of this thesis is allowable only for scholarly purposes, consistent with "fair use" as prescribed in the U.S. Copyright Law. Any other reproduction for any purposes or by any means shall not be allowed without my written permission. Signature Date University of Washington Abstract Liming effects on trace metal plant availability of a coarse-textured sewage sludge soil. by Sara Brallier Chairperson of the Supervisory Committee: Assistant Professor Robert B. Harrison College of Forest Resources A field study was conducted to determine the plant availability of trace metals from soils amended with 500 Mg ha-1 of municipal sewage sludge 16 years previously. The sludge-amended soil was adjusted with lime to four different pH levels ranging from an untreated pH of 4.6 to pH levels of approximately 5.8, 6.5 and 6.9. Crops selected for planting represented food crops important in the human diet and of differing metal uptake rates, including (i) bush beans (Phaseolus vulgaris L. cv. Seafarer), (ii) cabbage (Brasica oleracea L. v. capitata L. cv. Copenhagen market), (iii) carrots (Daucus carota L. cv. Toudo), (iv) corn (Zea mays L. cv. FR37), (v) lettuce (Lactuca sativa L. cv. Parris Island), (vi) potatoes (Solanum tuberosum L. cv. Kenebec), and (vii) tomatoes (Lycoperisicon esculentum L. cv. Burpee VF). With the exception of corn, crop yields were significantly reduced in the unlimed sludge-amended soils (pH 4.6). The edible portion of each crop was analyzed for Cd, Cu, Ni and Zn. Cabbage and lettuce tissue had Cd and Zn concentrations above suggested mean plant tolerance levels in the unlimed sludge-amended soil. All other crops and metals had concentrations below suggested tolerance levels. With the liming treatments, Cd and Zn concentrations were significantly reduced in the cabbage and lettuce to below the tolerance level. Liming also reduced total Ni in cabbage, lettuce and tomato fruit. Copper concentrations did not significantly change after liming. A sequential extraction method was used to determine relative solubility and availability of metals in the soils used for plant growth experiments. The relative solubilities of Cd, Ni and Zn were signifcantly reduced after liming the sludge-amended soil. The majority of the soil Cu was found in the insoluble and unavailable soil fractions before liming the sludge-amended soil and this did not change after liming. The results indicate Cd, Ni and Zn from the heavy sludge amendment remains extractable and plant available in acidic soils but is rapidly made unavailable when soil acidity is reduced even 16 years after sludge application. This study shows the benefit of pH adjustment in reducing metal uptake when sludge is applied at rates high enough to result in excessive nitrification and leaching with subsequent acidification of the soil. 4 TABLE OF CONTENTS List of Figures .................................................................................................... iii List of Tables ..................................................................................................... iv Acknowledgments ............................................................................................. v 1. INTRODUCTION .......................................................................................... 6 2. LITERATURE REVIEW ................................................................................ 8 2.1. Soil Factors Affecting Trace Metal Availability ................................ 8 2.1.1 Soil pH................................................................................ 9 2.1.2. Soil Organic Matter ........................................................... 10 2.1.3. Soil Cation Exchange Capacity ......................................... 11 2.1.4. Hydroxides of Iron, Manganese, and Aluminum................ 12 2.1.5. Amount and Type of Clay .................................................. 12 2.2. Metal Speciation Procedures .......................................................... 13 2.3. Plant Capacity for Trace Elements.................................................. 14 2.3.1. Food Chain Implications .................................................... 14 2.3.2. Plant Species .................................................................... 14 2.3.3. Plant Cultivars ................................................................... 14 2.3.4. Plant Parts and Age .......................................................... 15 2.4. Long Term Plant Availability of Sludge-borne Metals...................... 15 2.5. Effect of Sludge on Metal Concentration in Soil .............................. 16 2.6. Effect of Sludge Application on Metal Uptake by Plants ................. 17 2.7. Effect of Soil pH on Metal Activity in Sludge Amended Soils .......... 19 3. HYPOTHESIS AND STUDY OBJECTIVE .................................................... 26 4. METHODS AND MATERIALS ...................................................................... 27 4.1. Site Description ............................................................................... 27 4.1.1. Climate .............................................................................. 27 4.1.2. Soils .................................................................................. 27 4.2. Study Design .................................................................................. 27 4.2.1. Plant Analysis Procedure .................................................. 28 4.2.2. Soil Analysis Procedure .................................................... 29 4.3. Statistical Analysis .......................................................................... 30 5. RESULTS AND DISCUSSION ..................................................................... 32 5.1. Liming Effects on Metal Speciation and Soil Chemical Properties ............................................................................................... 32 5.1.1. Effects on Metal Speciation ............................................... 32 5.1.2. Effects on Soil Chemical Properties .................................. 33 5.2. Liming Effects on Plant Metal Uptake ............................................. 34 6. CONCLUSIONS ........................................................................................... 46 7. LITERATURE CITED ................................................................................... 48 APPENDIX 1: Plant Yield Data ......................................................................... 56 APPENDIX 2: Complete plant data analysis ..................................................... 59 ii LIST OF FIGURES Number Page 1. Forms of metals. ........................................................................................... 21 2. Stability of the ionic species of several trace metals as functions of soil pH.. ........................................................................................................ 22 3. The generalized effects of metal concentrations in nutrient solution on yield and metal content of plants. ............................................................ 23 4. Flowchart of the sequential extraction procedure used to determine the speciation of each metal in the soil samples. ......................................... 31 5. Soil metal speciation, in percent of total metal, by sequential extraction for the background (pH 5.9) and sludge-amended soils treated with lime to four different pH levels; pH 4.6, 5.8, 6.5 and 6.9. .......... 37 6. Trace metal concentrations in plant tissue grown in sludgeamended soil treated with lime to four different pH levels; pH 4.6, 5.8, 6.5 and 6.9. ........................................................................................... 38 iii LIST OF TABLES Number Page 1. Essentiality and effects of trace elements on plant and animal nutrition in terrestrial environment. ............................................................... 7 2. Sequential extraction techniques used to fractionate trace metals in soils and sludge-amended soils. .................................................................. 24 3. Approximate concentrations of trace elements in mature leaf tissue generalized for various species (ppm, dry wt.). ............................................ 25 4. Sequential extraction of soil metals, in percent of total metals, found at the surface soils (0-15 cm) in the back-ground (no sludge/no lime) and sludge-amended soils treated with lime to four different pH levels; pH 4.6, 5.8, 6.5 and 6.9. .............................................................. 40 5. Mean (±S.D.) soil elemental analysis of the background (no sludge/no lime) and sludge-amended soils treated with lime to four different pH levels; pH 4.6, 5.8, 6.5 and 6.9. ................................................ 42 6. Mean (±S.D.) metal concentration (dry wt.) in plant tissue grown in background (no sludge/no lime) and sludge-amended soils treated with lime to four different pH levels; pH 4.6, 5.8, 6.5 and 6.9. ...................... 43 iv ACKNOWLEDGMENTS I wish to express sincere appreciation to Dr. Robert B. Harrison, my supervisor, for his guidance in the course of my study and research. Special thanks are equally due to the other members of my committee; Dr. Charles L. Henry, Dr. Dale Cole, and Charles Treser, for their interest and assistance during the completion of this thesis. I also wish to express my appreciation to my fellow students whose criticism and suggestions were often invaluable to me. In addition, special thanks to my parents for their continuous love and support. This work was supported by the Regional W-170 Biosolids Research Group and funded by Northwest Regional Biosolids Committee. A special thanks to Dr. Rufus L. Chaney, USDA-ARS, and Dr. Terry J. Logan, Ohio State University, for their guidance and support in this research project. v 1. INTRODUCTION Composition of parent material and pedogenic processes in soil formation are usually the main factors determining metal concentrations in soil. Anthropogenic sources of trace metals are superimposed on these background levels. Sewage sludge, a byproduct of primary and secondary wastewater treatment processes, is one anthropogenic source. Sewage sludge typically contains organic matter and plant and animal nutrients primarily of domestic source, but may also contain trace metals from stormwater and industrial sources that enter the sewage system (Rappaport et al., 1988). The primary positive aspects of sewage sludge application to agricultural and forest land are generally considered to be the recycling of organic matter and nutrients and the increased plant and animal productivity associated with the addition of nutrients found in sewage sludge. Much research has focused the possible effects of trace metals and other persistent chemicals that may be present in sewage sludge from industrial sources. Logan & Chaney (1983) identified several elements as being of particular concern because elevated levels of these metals sometimes limit biotic productivity in nature. Those trace metals of concern include Cd, Cr, Cu, Pb, Hg, As, Mn, Ni, Se and Zn. Several of these metals are also necessary plant or animal nutrients, meaning their lack is detrimental to the health of associated plants or animals. Necessary trace elements include As, B, Co, Cr, Cu, F, Mn, Mo, Ni, Se, Sn, V and Zn (Table 1). Of the constituents most often found in sewage sludge, Cd, Cu, Ni and Zn may occur at concentrations, which, when applied to soils in excessive amounts, may depress plant yields, degrade the quality of food produced, or pose a threat to animal and human health through accumulation in plant tissues or direct ingestion of sludge-amended soil. 7 Table 1: Essentiality and effects of trace elements on plant and animal nutrition in the terrestrial environment (Source: Kabata-Pendias & Pendias, 1984). Essential or beneficial to__ Potential toxicity to Element Plants Animals Plants Animals As No Yes Yes Yes B Yes No Yes Co Yes Yes Yes Cr No Yes Yes Speciation important, Cr6+ very toxic; otherwise relatively nontoxic; carcinogenic Cu Yes Yes Yes Easily complexed in soils; narrow margin for plants F No Yes Yes Accumulative toxicity for plants and animals Mn Yes Yes <pH 5 Mo Yes Yes Ni No Yes Yes Yes Se Yes Yes Yes 4 ppm Narrow margin for animals; interacts with other trace metals Sn No Yes Yes Relatively nontoxic; very low uptake by plants V Yes Yes Yes Narrow margin and highly toxic in animals; carcinogenic 7 Phytotoxic before animal toxicity; may be carcinogenic Narrow margin, especially in plants Yes Relatively nontoxic; carcinogenic Wide margin; toxic in acid soils; among the least toxic 5-20 ppm Yes Comments Narrow margin for animals Very mobile in plants; relatively nontoxic; carcinogenic 8 Zn Yes Yes Wide margin; easily complexed in soils; maybe lacking in some diets; relatively nontoxic 8 2. LITERATURE REVIEW 2.1. Soil Factors Affecting Trace Metal Availability Plant availability of trace metals depends upon the physical and chemical properties of the soil-sludge layer. Factors affecting availability include the sludge metal chemical characteristics and loading rate, soil pH, CEC, redox potential, texture, and organic matter content (Verloo & Eeckhout, 1990; Logan & Chaney, 1983; Williams et al., 1980; Haghiri, 1974; Lagerwerff, 1972). Geochemically, a trace metal introduced into the soil may end up in one or more of the following forms (Figure 1): (1) Water Soluble: Exists in soil solutions as either free ions or soluble complexes with inorganic anions or organic ligands. (2) Exchangeable: Adsorption by electrostatic forces to negatively charged sites (for cations) or negatively charged sites (for anions) on clays, organic matter, hydrous oxides, or on amorphous materials. Exchangeable cations may be displaced by base cations commonly present in soil or sludge solutions. (3) Organic: Complexation with the organic fraction; chelated and/or organic bound. This category includes trace metal cations immobilized on or into living or recently dead biological material. The complexes may vary in stability from immediately mobile, easily decomposable, and moderately resistant to decomposition. Some of the organic material will be insoluble; some will have been flocculated or precipitated by complexing cations, notably iron or aluminum, but also by base cations like calcium or other trace metals. (4) Hydrous-oxide: Adsorption or coprecipitation with oxides, hydroxides, and hydrous oxides of Fe, Mn, and Al present as coating on clay minerals or as discrete particles. These oxides are rarely pure; they usually contain cations from each other, and probably trace metal cations as well. 10 (5) Carbonate: Carbonate precipitation in soils high in free CaCO3, bicarbonate, and alkaline in reaction. If precipitates do form, they usually contain more than one trace metal, and often form mixed crystals or mixtures of crystallites with the corresponding salts of major elements, usually calcium or iron. (6) Residual: Fixed or occluded within the crystalline lattices of soil minerals. Of those listed above, the first two forms are relatively mobile and plant available; while the last four are generally immobile but sometimes become mobile and plant available with time. Metal activity in the soil solution is generally considered to be the result of chemical equilibrium between the solution phase and solid phase among clay minerals, organic matter, hydrous oxides of Fe, Mn and Al, and soluble chelators (Adriano, 1986). The kinetics governing the chemical equilibrium of each metal species are complicated and not well understood. 2.1.1 Soil pH Of all the soil variables which have been reported to affect the solubility and availability of sludge-borne metals (organic matter content, cation exchange capacity, soil texture, pH, etc.) only pH has been shown to have consistent significant effect (Page et al., 1987; Logan & Chaney, 1983). The diversity of ionic species of trace metals and their various affinities to complex with inorganic and organic ligands make possible the dissolution of each element over a relatively wide range of pH. Figure 2 illustrates this change in ionic speciation for two trace metals (Zn and Cu) as a function of soil pH. Trace metals are generally more soluble and available under acid conditions due to the hydrolysis of hydroxy groups (OH) and the dissolution of solid phase minerals such as carbonates and phosphates (Lindsay, 1979; Ellis & Knezek, 1972). The pH dependent OH groups are primarily found on the edges and surfaces of the inorganic and organic colloids. The OH groups are attached to Fe and/or Al in the inorganic colloids (e.g., -Al-OH) and to the 10 11 CO groups in organic colloids (e.g., -CO-OH) Under moderately acid conditions, there is little or no charge on these particles, but as the pH increases, the hydrogen dissociates from the colloid OH group, and negative charges result (Brady, 1990). As the pH increases, more OH- ions are availabe to force the reaction to the right, and the negative charge on the particle surface increases. -Al-OH + -CO-OH + No charge OHOHalkaline soil solution -Al-O -CO-ONegative charge + H20 + H20 acid soil solution If the pH is lowered, OH- ions concentrations are reduced, the reaction goes back to the left, and the negativity is reduced. The relative mobility of some trace metals in soils as influenced by soil pH can be summarized as follows (Fuller & Alesii, 1979): (1) In acidic soil (pH 4.2 to 6.6), Cd, Hg, Ni and Zn are relatively mobile; As, Be and Cr are moderately mobile; and Cu, Pb and Se are slowly mobile. (2) In neutral to alkaline soils (pH 6.7 to 7.8), As and Cr are relatively mobile; Be, Cd, Hg and Zn are moderately mobile, and Cu, Pb and Ni are slowly mobile. 2.1.2. Soil Organic Matter Soil organic matter is often regarded as a major factor in the sorption of metals (Ellis & Knezek, 1972). This is because of both its cation exchange property and chelating ability. These complexes result from the binding of the metals to partially dissociated enolic ( -OH ), carboxyl ( -COOH ) and phenolic ( ) functional groups in the organic matter (Brady, 1990). Under very acid conditions, the negative charge is not very high because H+ is adsorbed on the surface of the functional groups. With a rise in pH, the H+ ions dissociate from first the carboxyl groups and then the enolic and phenolic groups. This leaves a greatly increased negative charge on the surface and 11 12 the H+ is replaced as shown below by other cations such as calcium and magnesium: Somewhat stable soluble and insoluble complexes between the metals and soil organic matter may form. Experimental results obtained for organic matter indicated that it has a scavenging action for trace metals that is far out of proportion to its own concentration (Tessler et al., 1979). However, organic matter has often shown a higher affinity for Cu and Pb than Cd, Ni and Zn with the stability of the metallo-organic complex increasing as pH increased from 3 to 7 (Kabata-Pendias & Pendias, 1984). 2.1.3. Soil Cation Exchange Capacity The CEC of soil is largely dependent on the amount and type of clay, organic matter, and Fe, Mn and Al oxides. These soil components usually have different cation exchange properties. Trace metal cations bind by electrostatic forces to the predominant negative charges on clays, organic matter and oxides. In general, the higher the CEC of soil, the greater the amount of metal a soil can accept without potential hazards (Korcak & Fanning, 1985). Numerous evidences indicate that CEC can best be viewed as a general, but imperfect, indicator of the soil components (i.e., clay, organic matter, and Fe, Mn and Al oxides) that limit the solubility of metals 12 13 instead of a specific factor in the availability of these metals (King, 1988; Latterell et al., 1976). 2.1.4. Hydroxides of Iron, Manganese, and Aluminum Considerable evidence indicates that hydroxides (Fe, Mn and Al) play a major role in the adsorption of trace metals in mineral soils (Keeney & Wildung, 1977). Metal oxides may occur as discrete crystalline minerals or as coatings on other soil minerals. Their capacity to adsorb or release trace metal cations from solution is controlled by pH and their crystalline structure. The adsorption of metals to oxides is related to the fact that most trace metals readily form hydroxyl species, while the more prevalent cations, Ca 2+ and Mg2+, do not. In general, the surfaces of hydrous Fe and Mn oxides are strong scavenging agents for trace metals. This is because the adsorption selectivity of trace metals is on the order of 103 to 106 over ions like Ca2+ and Mg2+. This permits selective adsorption of trace metals to the oxygen and hydroxyl surface groups of Fe, Mn and Al oxides. 2.1.5. Amount and Type of Clay The amount of clay in relation to the amount of silt and sand determines the soil texture, which in turn influences the CEC of soils. In general, the higher the clay content, the higher the CEC. The 2:1 type clay minerals usually have higher CEC than 1:1 types minerals. For example, montmorillonite (2:1 type) may have 80 to 100 meq/100 g compared to only 3 to 15 meq/100 g for kaolinite (1:1 type) (Brady, 1990). Korte et al. (1976) found that soil capacity for elements in the cationic form is best correlated with the surface area (amount of clay), while those present in anionic form are more strongly correlated with the free iron oxides in the soil. Of the trace metals present in cationic form, Cu and Pb are highly immobile in most soils, while Hg is relatively mobile. Of the metals in anionic form, soil capacity is decreased in the order Se, V, As and Cr. 13 14 2.2. Metal Speciation Procedures Several sequential chemical fractionation procedures designed to fractionate soil metals according to relative solubility have been proposed as a means to identify chemical forms of trace metals in soils. Some studies have also attempted to correlate these forms with mobility and plant availability (Lake et al., 1984). Sequential extraction methods have been used to partition metals into fractons defined as soluble, exchangeable or sorbed, organically complexed, precipitated, oxide-bound, occluded and residual, though the actual chemical forms by each extraction procedure are likely more complicated (Emmerich et al., 1982; Iyengar et al., 1981; Rappaport et al., 1988; Silviera & Sommers, 1977; Shuman, 1986; Sims, 1986). To estimate these fractions, different extracting solutions have been used. Table 2 lists some of the extractants used to fractionate metal species From Table 2, it can be noted that there are a wide diversity of extractants used to fractionate metal species, although experimental results are often contradictory and inconsistent. This makes comparison or prediction of results difficult. Experimental conditions must, therefore, be accurately defined: namely, the nature of the counter cation, the nature of the anion, ionic strength, and the pH and soil solution ratio values (Calvet & Bourgeois, 1990; Lake et al., 1984). The use of soil extractants to predict plant availability of metals has been a subject of a great deal of attempts to correlate with plant uptake studies, but many problems have been seen with these studies (Giordano et al., 1979). Such simple extraction's may be useful under certain conditions; however, such as comparing similar soils, comparing treatment effects on the same soil, etc. Today, there are additional analytical techniques used for speciation of metals to help alleviate the inconsistencies found with sequential extraction procedures. They are divided into two groups: (i) Chromatographic techniques; (ii) Multipurpose computer programs; and (iii) kinetic chemical methods (i.e., 8-hyroxyquinoline extraction) Chromatographic techniques include ion-exchange, adsorbent resin, gel permeation, and high performance liquid chromatography (HPLC). Multipurpose computer programs include 14 15 programs such as GEOCHEM. Computer programs are used for speciation computations, predicting the metal species likely to occur in the solution phase, on the basis of known chemical equilibria and properties of the soils concerned. Analytical results, in combination with computer modeling, can give fairly accurate predictions of the different metal forms. Computer modeling, however, is useful only for setting limits on speciation and needs to be run in conjunction with analytical procedures to validate their results (Verloo & Eeckhout, 1990; Lake et al., 1984). 2.3. Plant Capacity for Trace Elements 2.3.1. Food Chain Implications One of the basic environmental problems relates to the quantities of accumulated metals in plant parts used as food. The generalized effects of metal concentrations in nutrient solution on yield and metal content of plants are shown in Figure 3. The approimate concentrations of metal toxicity or deficiency found in various plant species are listed in Table 3. In addition to the various soil factors previously discussed that can influence plant availability of trace metals, the following factors can also affect the ability of plants to accumulate trace metals: plant species, plant cultivars, and plant parts and age. 2.3.2. Plant Species Crops differ widely in their sensitivity to excess trace metals. In general, leafy vegetables are the greatest accumulators of soil trace elements. Crops differ considerably in their sensitivity to individual trace metals. Generally, at about pH 5.5 to 6.5, Cu could be twice as toxic as Zinc, and Ni four times as toxic as Zn at equilvalent concentrations (Adriano, 1986). 2.3.3. Plant Cultivars Differences in uptake of trace metals among cultivars have long been established. Investigators recently found differential uptake and translocation 15 16 of Cd and Zn among lettuce and corn cultivars (Giordano et al., 1979; Hinesly et al., 1979); and differential Cd uptake among soybean cultivars (Boggess et al., 1978). This evidence indicates there may be a genetic basis of differential translocation of metals within plants. 2.3.4. Plant Parts and Age It is common observation that trace elements are not uniformly distributed among plant tissues. In general, the seeds (or grain) contain lower concentrations of most trace elements than do the vegetative tissues. Thus, grain crops are very likely to contribute smaller amounts of trace elements to the diet than do leafy vegetables. Garland et al. (1981) suggested that the distribution of elements in various vegetative tissues (exclusive of stems) is a characteristic of xylem transport and that the ultimate concentration of an element in a specific tissue is related to the flux of transpirational water lost through evapotranspiration and the duration of this process for specific tissues. Age also has an effect on trace element concentration in plants. Data indicate a tendency for trace element concentrations (Cd, Cr, Cu, Mn, Pb and Zn) in fescue leaves to decrease during the growing season (Boswell, 1975). 2.4. Long Term Plant Availability of Sludge-borne Metals It has been suggested some metals (Zn, Ni and Cd) might increase in solubility during sludge decomposition in the soil (Dudley et al., 1986; Behel et al., 1983; Chang et al., 1983; Holtzclaw et al., 1978), however, a lowering of metal solubility and uptake has typically been demonstrated in the results of research projects (Emmerich et al., 1982). It has been observed from long term sludge studies that plant availability of sludge-borne metals is highest during the first year after sludge is applied (Bidwell & Dowdy, 1987; Chang et al., 1987; Hinesly & Hansen, 1984). This is in contrast to the long-held belief that once the sludge applied organic matter is oxidized complexed metal will be released and plant uptake will increase (Beckett et al., 1979). Additionally, 16 17 this belief is not supported by studies of sludge chemistry which indicate that digested sludge in addition to being 50% organic matter is 50% inert inorganic mineral forms (including Fe and Al oxides, silicates, phosphates, and carbonates) that are reactive with the metals and environmentally stable (Essington & Mattigod, 1991; McCalla et al., 1977). 2.5. Effect of Sludge on Metal Concentration in Soil The concentration of Zn, Cd, Cu, Ni, Mn, Ca, Mg, Na, K, PO43--P, SO42--S and Cl in soil solution typically increases with sludge application rates, particularly with multiple sludge applications at high rates in fine-textured soils. In general, soil solution concentrations of Cd, Cu, Ni, Pb and Zn are low at all sludge application rates due to the retention of metals in mineral and organic complexes. However, soluble Cu and Ni concentrations in soil treated with sludge at rates of 800 Mg ha-1 were markedly higher than those receiving lesser amounts of sludge (Behel et al., 1983). Application of 200 Mg ha-1 of sludge at one time resulted in higher levels of soluble Zn, Cd, Cu, PO43--P and Cl and higher pH than a similar total amount of sludge applied in a consecutive annual addition of 50 Mg ha-1 yr-1. Rappaport et al. (1988) found that DTPA extractable (a sparingly soluble form) of Cd, Cu, Ni and Zn increased with rate of sludge application. The order of DTPA extractable metals concentration in the soil was Cu > Zn > Ni > Cd. Extractions with DTPA have been shown to be somewhat correlated with plant uptake (Singh & Narwal, 1984; Street et al., 1977; Bingham et al., 1975). However, in some cases, plant uptake did not correlate with DTPA extraction (Giordano et al., 1979). It was further reported that the extractability of metals from the sludge-amended soil were controlled more by the properties of the sludge rather than the soil properties. Giordano et al. (1979) reported that the concentration of DTPA extractable Cd, Zn, Cu, Pb and Ni in sludge-amended soil increased apparently because of progressive decomposition of the organic matrix, though the water solubility decreased with time. 17 18 Liming can also have an effect on the metal solubility in soil. This is because there is a greater complexation of metals by soluble organic carbon with liming (Dudley et al., 1986), and for most metals, decreased solubility through inorganic processes as well. Campbell & Evans (1987) reported that both H+ and humic acid concentration influence the percentage of soluble Pb and Cd. Lead is largely bound by humic acids and to a lesser extent by carbonate but if the concentration of Ca increases in the system, more Pb will bind with the humic acids. Cadmium binding to humic acids is much lower than that of Pb, which will result in a higher availability of Cd than that of Pb in sludge-amended soils, though Cd is strongly retained in many circumstances. Over time, it appeared that Cd, Ni and Zn shifted toward less-soluble form, while Cu remains in a relatively insoluble organic form (Emmerich et al., 1982. 2.6. Effect of Sludge Application on Metal Uptake by Plants Various studies have reported that plants absorb substantial amounts of additional metals from sludge-amended soils. In a recent pot study, a linear relationship was observed between the total Cd concentration of 17 anaerobically digested sludges and Cd concentration of Sudax when the sludges were applied at a constant Cd rate (Jung & Logan, 1992). Metal accumulation by plants depends upon plant species, sludge and soil physical and chemical properties as well as the metal concentrations and forms. Metal contents of vegetative tissues, such as leaves and stems, have generally been reported to be higher than those of the fruiting, root and tuber tissues (Vlamis et al., 1985; Bingham et al., 1975; Dowdy & Larson, 1975). It has been observed that sludge with low metal concentrations have lower plant uptake at the same metal loading than do sludges with higher metal concentrations (Logan, 1989; Corey et al., 1987). In order to see substantial plant uptake of metals where the source of those metals is sludge, extremely high application rates are typically necessary, usually >100 Mg ha -1 of sludge with high metal levels. Lower rates of sludge or the application of sludge with low metal content results in no additional metal uptake by most plants, with 18 19 the exception of certain metal accumulators such as lettuce or swiss chard. Dowdy & Larson (1975) observed that in most edible tissue, trace metal accumulations did not increase more than two or three fold as a result of amending the soil with 450 Mg ha-1 of sludge. This would result in the average soil plow layer consisting of about 25% sludge. Reductions in metal uptake were probably the result of the binding of free metals in soil by sludge organic matter. These observations have led some researchers to suggest that the uptake of trace metals by plants is a function of soil organic matter content, cation exchange capacity, pH, soil texture, and Fe, Mn and Al oxide (King, 1988; Korcak & Fanning, 1985; Singh & Narwal, 1984; Chang et al., 1983; King & Dunlop, 1982; Haghiri, 1974). In general, it has been observed that the uptake of most metals, particularly Cd, increases with decreasing soil pH (Gerritse et al., 1982; Dijkshoorn et al., 1981; Giordano et al., 1979; Bingham et al., 1975). However, the competition of the H+ ion with the Cd2+ ion for plant uptake at lower pH levels may explain why the uptake is not as great as the increase in solubility would indicate (Hatch et al., 1988), and why in some cases, no decrease or even an increase in uptake was noted with liming (Pepper et al., 1983; Hemphill et al., 1982; King & Dunlop, 1982). It is speculated that in some soils, particularly those with high amounts of organic matter, the mechanisms of metal retention may be quite different than in soils where inorganic retention mechanisms dominate. The presence of nitrogen in sludge can also enhance the uptake of Zn, Cd, Cr, Pb and Ni by plants in sludge-amended soils either because of the increased growth or due to mobilization of metals from acidification due to nitrification (Chang et al., 1983; Giordano & Mortvedt, 1976). The mineralization of organic matter to nitrate results in the production of 2 H+, which can greatly acidify soil as follows: 19 20 It is important to note that most of the available studies with sewage sludge were conducted with sludges containing metals at levels higher than the median concentration in current U.S. sludges (EPA, 1990). The cited studies were either deliberately conducted with high metal sludges (e.g., the Chicago sludge of the 1970's which had a Cd concentration of approximately 200 mg/kg) or simply reflect the higher metal concentrations that were present prior to industrial pretreatment programs. As not all studies give the metal concentration of the sludges used, it is not possible to determine sludge metal concentration for all studies. 2.7. Effect of Soil pH on Metal Activity in Sludge Amended Soils Some researchers found that pH reduction in the sludge-soil layer increased the solubility of metals and increased their potential for movement in the soil profile. Boswell (1975) applied sewage sludge of pH 5.6 to a soil that had been limed to a pH of 6.2. Hinesly et al. (1972) started with a soil pH of 5.6, which at the end of two years had dropped to 4.9. Fuller & Alesii (1979) observed that at lower soil pH levels and higher concentration of metals in the sludge, there was more rapid movement of metals through the soil. The amount of Pb, Ni, Cd, Cd and Zn retained by soil depended upon the pH of the soil, nature of the clay and the chemical nature of organic compounds present (Harter, 1983; Hatton & Pickering, 1980). King (1988) observed that the relative retention of metals by most soils was in the order of Pb > Cu > Cr > Zn > Ni > Co > Cd. Sorbed and nonexchangeable Cd, Co, Cu, Ni and Zn were better related to pH than to any of the other soil properties. Sorbed and nonexchangeable Pb, Cr and Sb 20 21 were more related to sand, clay or Fe oxide content. Additionally, retention in organic soils were superior to retention in nearly all of the mineral soils. Lowered pH of the soil profile beneath the sludge soil layer was attributed to mineralization and nitrification of sludge organic nitrogen (Chang et al., 1987; Emmerich et al., 1982). With time intense nitrification and the lowering of pH may be conducive to the movement of metals in the soil profile. However, no direct evidence of this has been actually observed, and there is the potential for increased retention due to a buildup of organic matter in the soil from the additional nitrogen and increased microbial and plant activity as well. 21 22 Figure 1: Forms of trace metals in soil solutions. 22 23 Figure 2: The hydrolysis species of Zn2+ and Cu2+ as functions of soil pH in equilibrium with soil Zn or Cu (Source: Kabata-Pendias & Pendias, 1984). 23 24 Figure 3: The generalized effects of metal concentrations in nutrient solution on yield and metal content of plants (Source: Kabata-Pendias & Pendias, 1984). 24 25 Table 2: Sequential extraction techniques used to fractionate trace metals in soils and sludge-amended soils (Source: Lake et al., 1984). Metal Studied Silviera & Alloway et Sommers al. (1979) (1977) Cd,Cu, Cd Pb, Zn Metal Form: Extractant Used: Soluble H2 O Source Exchangeable KNO3 Petruzzelli Emmerich Schalscha et al. (1981) et al. (1982) et al. (1980; 1982) Cd, Cu, Ni, Cd, Cu, Ni, Cr, Cu, Mn, Pb, Zn Zn Ni, Zn Soon & Bates (1982) Cd, Ni, Zn H2 O H2 O CH3CO2- KNO3 NH4 CaCl2 KNO3 KNO3 Adsorbed KNO3 Deionized H2 0 NaOH Na4P2O7 (CH3 CO2)2Cu NaOH Carbonate HNO3 Precipitated Na2-EDTA EDTA HNO3 Na2EDTA Sulfide Precipitated HNO3 HNO3 Available K4 P 2 O7 Cd,Cu,Ni Pb, Zn NaF Ion Exchange Organically Bound Sposito et al. (1982) DTPA DTPA Occluded HONH2HCL Residual HNO3** HNO3 ** * Cu-Ox consists of oxalic acid (CO2H)2 and oxalate (CO2NH4)2 ** Residual forms determined by subtracting sum of extracted forms from total metal concentration. 25 HNO3 26 Table 3: Approximate concentrations of trace elements in mature leaf tissue generalized for various species (ppm, dry wt.) (Source: Kabata-Pendias & Pendias, 1984). Deficient, if less than the stated amounts of Sufficient or Excessive or Element essential elements normal toxic Ag As ------------- 0.5 1 - 1.7 5 - 10 5 - 20 B Ba Be Cd Co Cr Cu F Hg 5 - 30 ------------------------------2-5 ------------- 10 - 200 ------<1 - 7 0.05 - 0.2 0.02 - 1 0.1 - 0.5 5 - 30 5 - 30 ------- 50 - 200 500 10 - 50 5 - 30 15 - 50 5 - 30 20 - 100 50 - 500 1-3 Li Mn Mo Ni Pb Se Sn Sb Ti ------15 - 25 0.1 - 0.3 ------------------------------------- 3 20 - 300 0.2 - 1 0.1 - 5 5 - 10 0.01 - 2 ------7 - 50 ------- 5 - 50 300 - 500 10 - 50 10 - 100 30 - 300 5 - 30 60 150 50 - 200 Tl V Zn Zr ------------10 - 20 ------- ------0.2 - 1.5 27 - 150 ------- 20 5 - 10 100 - 400 15 Note: Values are not given for very sensitive or highly tolerant plant species. 26 3. HYPOTHESIS AND STUDY OBJECTIVE The objective of this study was to determine if pH has an effect on the extractability and plant availability of Cd, Cu, Ni and Zn in a soil amended with a single high application of sewage sludge sixteen years earlier. The following two hypothesis were developed to test this theory: Hypothesis 1: Extractability of the Cd, Cu, Ni and Zn is a function of its free ion concentration (water soluble and exchangeable form). This will be done by measuring the solubility of Cd, Cu, Ni and Zn is a function of pH in a sludge-amended soil treated treated with lime to four different pH levels. By changing the soil pH, it can be observed if pH can still affect the free ion concentration of Cd, Cu, Ni and Zn in a soil amended with sewage sludge sixteen years earlier. Hypothesis 2: Plant availability of Cd, Cu, Ni and Zn is a function of its free ion concentration. This will be done by measuring the plant availability of Cd, Cu, Ni and Zn primarily by changing the relative solubility of metal fractions and measuring the concentrations of metals in plant tissue grown in a sludge-amended soil treated with lime to four different pH levels and comparing uptake to the concentration of metal fractions. By changing the soil pH, it can be observed if the availability of soil Cd, Cu, Ni and Zn can still be affected sixteen years after sludge application. 4. METHODS AND MATERIALS 4.1. Site Description This field study was conducted at the University of Washington's Charles Lathrop Pack Forest Research Station, located approximately 100 km south of Seattle near Eatonville, Washington. 4.1.1. Climate The maritime climate at Pack Forest is mild and wet with a mean annual temperature of 9° C. Mean annual precipitation is 114 cm with over 75% of the precipitation falling between October and April. Winter temperatures are mild enough that nearly all of the precipitation falls as rain. 4.1.2. Soils The soil, prior to the application of sewage sludge, is classified as a Barneston series by the U.S.D.A Soil Conservation Service (1979). Barneston soil is a somewhat excessively drained gravelly sandy loam (mesic Dystric xerochrepts) that formed in glacial outwash under conifers along the foothills of the Cascades. In a typical undisturbed profile a mat of extremely acid (pH 4.2), undecomposed needles and wood overlies a 5-inch, very dark brown and very dark grayish brown, very strongly acid (pH 4.4) gravelly sandy loam surface layer. The subsoil, to a depth of 13 inches, is a dark yellowish brown, strongly acid (pH 4.8-5.2) coarse gravelly sandy loam. The substratum, to a depth of more than 60 inches, is a brown, medium acid (pH 5.8) very gravelly sand. 4.2. Study Design The site was amended in 1975 with a single high application (500 Mg ha 1) of anaerobically digested dewatered sewage sludge from the Municipality of Metropolitan Seattle (METRO) wastewater treatment plants. In the 29 summer of 1991, the sludge-amended soil was divided into four 3 x 11-m plots and planted with vegetable crops. One 3 x 11-m sludge-amended plot was not treated with lime to serve as a control with an initial pH level of approximately 4.6. The remaining three sludge-amended plots were treated with hydrated lime, Ca(OH)2, at rates of 8, 15, and 22 Mg ha-1 resulting in a soil pH of approximately 5.8, 6.5, and 6.9, respectively. To quantify backgound soil and plant metal concentrations for comparison purposes only, four untreated 3 x 11-m plots (unsludged and unlimed) were used with soil pH levels of approximately 5.9. These four untreated plots were located approximately 30-m from the treated sludge-amended plots. Vegetable crops selected for planting represented foods important in the human diet and showing differing metal uptake rates: (i) bush beans (Phaseolus vulgaris L. cv. Seafarer), (ii) cabbage (Brasica oleracea L. v. capitata L. cv. Copenhagen market), (iii) carrots (Daucus carota L. cv. Toudo), (iv) corn (Zea mays L. cv. FR37), (v) lettuce (Lactuca sativa L. cv. Parris Island), (vi) potatoes (Solanum tuberosum L. cv. Kenebec), and (vii) tomatoes (Lycoperisicon esculentum L. cv. Burpee VF). 4.2.1. Plant Analysis Procedure During the growing season, all plots were fertilized with optimum amounts of commercial NPK fertilizer (10:10:10; without micronutrients) to ensure nutrient availability was not limiting. Plant height at maximum vegetative growth stage, and total above ground biomass (wet weight basis) were measured at time of harvesting for the cabbage, corn and lettuce. Total harvestable biomass (total wet weight of heads of lettuce and cabbage, tubers of potato, fruit of tomato, bean pods, and corn stalk at tasseling above the 2nd leaf) was measured at the time the edible tissue was sampled for analysis. The edible portion of the plant tissues were dried at 70 °C to constant weight, then ground to <2-mm in a stainless steel Wiley mill for analysis. Total Cd, Cu, Ni and Zn in plant tissue samples were determined using an HNO3-H2O2-HCL acid digestion (EPA Standard Method 3050) and 29 30 inductively coupled argon plasma spectroscopy (ICP; Thermo Jarrel Ash ICAP 61E, Thermo Jarrel Ash, Franklin, MA). 4.2.2. Soil Analysis Procedure Three soil core samples were collected from the Ap horizon (0-15 cm) in each plot. The soil samples were analyzed for pH, total Cd, Cu, Ni and Zn, trace metal speciation (Henry & Harrison, 1992), CEC, total N and organic C. Samples were air dried and screened to < 2-mm prior to physical and chemical analysis. Soil pH was determined in a 1:2 soil-to-deionized water suspension after equilibration for 1 hour (Corning pH Analyzer 250, Corning, NY). Sequential extractions were used to fractionate the trace metals in the soil samples (Henry & Harrison, 1992). The extractions were done in sequence on the same soil sample using the following extraction technique (Figure 4): (1) deionized water extraction for soluble metals (Msoluble); (2) 1.0M MgCl2 extraction for exchangeable forms (Mexchangeable); (3) 0.1M Na4P2O7 extraction for organically bound forms (Morganic); and (4) HNO3-H2O2-HCL acid digestion of the residual fraction (Mresidual). Metal concentrations in solutions were measured on an ICP. Results for each fraction were reported as the percent of the total soil metal. Total metals were determined using the HNO3-H2O2-HCL acid digestion (EPA Standard Method 3050). Cation exchange capacity was determined using the following steps: (1) soil was saturatedwith 1.0 M NH4Cl, (2) soil pore water was removed with two ethanol leachings to remove soluble NH4+, (3) NH4+ was displaced with 1.0 M KCl, and (4) NH4+ was analyzed on a Technicon Autoanalyzer (Technicon Autoanalyzer II, Tarrytown, NY). Total N was determined by digesting soil samples using a modified Li2SO4-H2SO4 digestion method (Parkinson & Allen, 1975) and analyzed on an 30 31 Autoanalyzer. Total organic C content was determined by dry combustion (LECO model 761-100 C Determination: LECO Corp., Joseph, MI). 31 32 4.3. Statistical Analysis The experiment was treated as a 4 x 3 factorial with a minimum of three replicates per cell (four treatment levels; three cells within each treatment level, and three replicates within each cell). An analysis of variance (ANOVA) was used to test the differences of means in plant metal concentrations at each pH level in the sludge-amended soil (SYSTAT, 1990). The unsludged and unlimed plots were used to quantify background concentrations for comparison purposes only. 32 33 Figure 4: Flowchart of the sequential extraction procedure used to determine the speciation of each metal in the soil samples. 33 5. RESULTS AND DISCUSSION 5.1. Liming Effects on Metal Speciation and Soil Chemical Properties 5.1.1. Effects on Metal Speciation Figure 5 shows the percentage of metals in the four fractions of the sludge-amended soil at each pH level. Background soil fractions by sequential extraction are included for comparison purposes. Based on the observation that the water soluble and exchangeable metal fractions are often plant available (Brady, 1990), the water soluble + exchangeable metal frations (Msol+exch) were used to indicate relative solubility of metal in soils. Liming the sludge amended soil significantly decreased Cdsol+exch, Nisol+exch and Znsol+exch. Reductions were 45, 90 and 95%, respectively. Liming resulted in only minor changes in Cusol+exch (<2%). For example, Cdsol+exch, Nisol+exch and Znsol+exch fractions in the unlimed, sludge-amended soil were high (28%, 27% and 30% respectively), while the limed sludge-amended soil Cdsol+exch, Nisol+exch and Znsol+exch were much lower (15%, 2% and trace, respectively). The Cusol+exch in the unlimed sludge-amended soil was only 2% of total, while the limed, sludge-amended soil had only trace concentrations. Similar studies of the effects of liming showed Zn exchangeable was decreased by an average of 60% to 95%, depending on the soil type, when the soil pH was increased from 4.8 to 7.5 (Sims, 1986; Shuman, 1986). Alloway et al. (1979) found the distribution of Cd in a sludge-amended acid soil (pH 5.9) for the water soluble and exchangeable fractions totaled 22%, organic bound 45%, hydrous-oxide bound 20%, and the rest of the metal was in the residual form. Street et al. (1978) observed that watersoluble Cd decreased as much as 50% as pH was increased from 5.0 to 7.8. This study also found a similar distribution of Cd in the acidic unlimed sludgeamended soil (Table 4 and Figure 5). As the pH was increased, the proportion of Cd in the water soluble plus exchangable fraction decreased from 29% to 15% while the residual fraction increased from 35% to 55%. 35 This decreased solubility of Cd in soils is associated with the formation of CdCO3 and Cd3(PO4)2 with increasing pH (Street et al., 1977). Elevated pH can also change the nature of the exchange sites by hydrolyzing or precipitating Al3+ ions that occupy the exchange sites, thus creating more exchange sites (Adriano, 1986). With the possible exception of Pb, Cu has been found to be the most strongly adsorbed than Cd, Ni and Zn on organic matter, Fe and Al oxides and oxyhydroxides (McBride, 1989). The stability of the Pb and Cu metalloorganic complex often increasing as pH increased from 3 to 7 (KabataPendias & Pendias, 1984). In a study conducted by McLaren & Crawford (1973), from 20 to 50% of the Cu in 24 soils of diverse types occured in organically bound forms. In soil sorption studies, the amount of Ni retained was also dependent upon the pH of the soil, with retention increasing with increasing pH (Harter, 1983; Gerritse et al., 1982). Berrow & Burridge (1990) found increasing the soil pH from 4.5 to 6.5 decreased the plant available fraction of Ni, thus decreasing the Ni content of oats grain by a factor of about 8. Similar studies of the effects of liming showed exchangeable Zn was decreased by an average of 60% to 95%, depending on the soil type, when pH was increased from 4.8 to 7.5 (Sims, 1986; Shuman, 1986). Zinc adsoprtion by carbonates, precipitation of Zn hydroxide or carbonates, or formation of insoluble calcium zincate are believed partly responsible for Zn unavailability in alkaline soils (Farrah & Pickering, 1977; Shuman, 1979; Bingham et al., 1964). 5.1.2. Effects on Soil Chemical Properties Soil pH was reduced in the sludge-amended soil, probably due to the significant nitrification that followed the sludge application (Table 5). For instance, the unamended soil had an average pH level of 5.9, while the sludge-amended, unlimed soil had a pH level of 4.6. Total N and organic C were increased in the 0-15 cm soil horizon as a result of the addition of sewage sludge. For instance, total C was 67 vs 184 35 36 mg g-1 and total N was 3.4 vs. 12.9 mg g-1 in the unamended and sludgeamended soils, respectively. Total N and C concentrations were not significantly affected by liming (Table 5). Results also indicate CEC was significantly higher in the unamended soil compared to the unlimed sludgeamended soil (18.1 vs 11.6 cmolc kg-1, respectively). Though the large amendment of organic matter would normally increase CEC, the effective CEC of this soil was apparently greatly reduced by the reduction in soil pH. The liming treatments increased CEC to levels similar to background soils (Table 5). 5.2. Liming Effects on Plant Metal Uptake The most consistent trends in plant metal concentrations observed in this study occurred with Cd, Ni and Zn (Table 6). In general, metal concentrations in plant tissue grown in the sludge-amended soil were in the order (Table 6 , Figure 6 and Appendix 2): bush bean foliage > corn stalk > cabbage > lettuce > potato peels > peeled potato tuber = tomato fruit Tissue Cd, Ni and Zn concentrations in cabbage and lettuce tissue were significantly reduced (p<0.01) in the limed sludge-amended soils to below suggested mean plant tolerance levels (in foliage) of 3, 50 and 300 mg kg-1 (Figure 6). Copper concentration; however, was not reduced by liming. In the peeled potato tuber and tomato fruit, tissue Cd, Ni and Zn concentrations were significantly reduced by treatment with lime (p<0.01). While Cu concentration generally did not change after liming in the peeled potato tuber, concentration in the tomato fruit did appear to reach deficiency levels at the highest liming rate (pH 6.9). Ni concentration was significantly reduced (p<0.01) in the tomato fruit but was not reduced in the peeled potato tuber. Cadmium, Ni and Zn concentrations in the potato peels were all significantly 36 37 reduced (p<0.01) by treatment with lime, while Cu concentration was not reduced. In general, metal concentrations in each of the different plant tissues had the following trends (Table 6): Cadmium concentration in the plant tissue after liming was in the order: bush bean foliage > corn stalk > lettuce > cabbage, potato peel > peeled potato tuber, tomato fruit; Copper concentration was in the order: bush bean foliage > potato peel > lettuce > cabbage, corn stalk > peeled potato tuber, tomato fruit; Nickel concentrations was in the order: bush bean foliage, cabbage > corn stalk > lettuce > peeled potato tuber, tomato fruit; and Zinc concentration was in the order: bush bean foliage > corn stalk > lettuce > cabbage > potato peel > peeled potato tuber > tomato fruit. The above-ground yield for the cabbage and lettuce was significantly reduced in the unlimed sludge-amended soil (Appendix 1). The aboveground yield for corn was not reduced in the unlimed sludge-amended soil and was unaffected by liming. Total above ground yield was not measured for the bush beans. The bush beans also did not produce any legumes in the unlimed sludge-amended soil, and consequently, I was unable to compare the effect of liming on the uptake of metals in legumes. Total above ground yields were not measured for the potato and tomato crops; however, total fruit and tuber yield was increased after liming. These results agree with studies suggesting that Cd, Ni and Zn in soils may be made unavailable to plants by the application of hydrated lime and, consequently, phytotoxicity prevented (Bingham et al., 1979; MacLean & Dekker, 1978: Chaney et al., 1977). Soil pH appears to be the most important soil property that determines plant availability for Cd, Ni and Zn. The higher solubility of these three metals in the unlimed sludge-amended soil was in agreement with recognized increasing solubility of the metals with increasing soil acidity (Logan & Chaney, 1973). Cadium uptake by plants was found to almost always to decrease with decreasing pH (Adriano et al., 1982; Bingham et al., 1979; Mahler et al., 1978). In studies of Zn uptake at different pH levels, uptake declined sharply when soils were limed from pH 37 38 4.3 to above 5.6 (Friesen et al., 1980; Gupta et al., 1971). Liming is effective because these metals probably form an insoluble precipitate with hydroxides, carbonates and phosphates and thus becomes unavailable to plants (Reddy & Patrick, 1977). Copper concentrations remained more stable and generally were unaffected by changes in pH. This was in agreement with the general affinity of Cu to form stable complexes with organic matter (Harter, 1983; Ellis & Knezek, 1972). A number of studies have shown that Cu in soil solutions, especially at higher pH, exists primarily in a form complexed with organic matter which is relatively unavailable to plants (Hodgson, 1963). In fact, King & Dunlop (1982) concluded that high organic matter content in soils can substitute for high pH in immobilizing metals and thus sewage sludge can be applied to organic soils that have pH values lower than the currently suggested value of pH 6.5 for metal application from municipal sewage sludges. 38 39 Figure 5: Soil metal speciation, in percent of total metal, by sequential extraction for the background (pH 5.9) and sludge-amended soils treated with lime to four different pH levels; pH 4.6, 5.8, 6.5 and 6.9. 39 40 Figure 6: Trace metal concentrations (mg kg-1, dry wt.) in plant tissue grown in sludge-amended soil with treated with lime to four different pH levels; pH 4.6, 5.8, 6.5 and 6.9. 40 41 Figure 6 (con't): Trace metal concentrations (mg kg-1, dry wt.) in plant tissue grown in sludge-amended soil with treated with lime to four different pH levels; pH 4.6, 5.8, 6.5 and 6.9. 41 42 Table 4: Sequential extraction of soil metals, in percent of total metals, found at the surface soils (0-15 cm) in the back-ground (no sludge/no lime) and sludge-amended soils treated with lime to four different pH levels; pH 4.6, 5.8, 6.5 and 6.9. CADMIUM Water Amendment Soluble Exchangeable Organic Residual ----------------------------------%----------------------------No Sludge/No Lime - pH 5.9 ND * 31.6 31.6 36.7 Sludge/No Lime - pH 4.6 1.31 27.7 36.3 34.6 Sludge/Lime - pH 5.8 1.28 24.8 35.3 38.6 Sludge/Lime - pH 6.5 ND 18.0 30.3 51.7 Sludge/Lime - pH 6.9 ND 15.3 29.5 55.3 COPPER Water Amendment Soluble Exchangeable Organic Residual ----------------------------------%----------------------------No Sludge/No Lime - pH 5.9 8.58 ND 64.1 27.3 Sludge/No Lime - pH 4.6 ND 1.88 57.8 40.2 Sludge/Lime - pH 5.8 ND ND 58.1 41.4 Sludge/Lime - pH 6.5 ND TR ** 45.4 53.9 Sludge/Lime - pH 6.9 TR TR 43.4 55.7 NICKEL Water Soluble Exchangeable Organic Residual ----------------------------------%----------------------------No Sludge/No Lime - pH 5.9 3.96 3.96 ND 92.1 Sludge/No Lime - pH 4.6 1.21 26.9 14.9 57.0 Sludge/Lime - pH 5.8 1.08 16.7 18.3 64.0 Sludge/Lime - pH 6.5 1.33 5.57 17.2 75.9 Sludge/Lime - pH 6.9 1.37 1.87 17.6 79.1 Amendment 42 43 Table 4 (con't): Sequential extraction of soil metals, in percent of total metals, found at the surface soils (0-15 cm) in the background (no sludge/no lime) and sludge-amended soils treated with lime to four different pH levels; pH 4.6, 5.8, 6.5 and 6.9. ZINC Water Amendment Soluble Exchangeable Organic Residual ----------------------------------%----------------------------No Sludge/No Lime - pH 5.9 ND 10.1 11.9 78.0 Sludge/No Lime - pH 4.6 1.30 29.6 47.7 21.5 Sludge/Lime - pH 5.8 TR 14.2 58.0 27.5 Sludge/Lime - pH 6.5 ND 3.35 49.4 47.2 Sludge/Lime - pH 6.9 ND TR 50.0 49.7 * ND = nondetected ** TR = trace 43 44 Table 5: Mean (±S.D.) soil elemental analysis of the background (no sludge/no lime) and sludge-amended soils treated with limed to four different pH levels; pH 4.6, 5.8, 6.5 and 6.9. All soil samples were collected from a depth of 0 to 15 cm. Amendment pH %N %C CEC (cmolc kg-1) No Sludge/No Lime 5.9 (0.1) 0.34 (0.04) 6.74 (1.1) 18.1 (2.9) Sludge-No Lime 4.6 (0.1) 1.29 (0.18) 18.4 (2.6) 11.6 (1.0) Sludge-Limed 5.8 (0.1) 1.18 (0.27) 16.9 (3.9) 19.3 (2.1) Sludge-Limed 6.5 (0.3) 1.19 (0.11) 17.0 (1.5) 19.6 (2.5) Sludge-Limed 6.9 (0.2) 1.17 (0.17) 16.8 (2.5) 23.0 (2.3) 44 45 Table 6: Mean (±S.D.) metal concentration (mg kg-1, dry wt.) in plant tissue grown in background (no sludge/no lime) and sludge-amended soils treated with lime to four different pH levels; pH 4.6, 5.8, 6.5 and 6.9. BEAN LEAF Amendment Cd Cu Ni Zn -------------------------- mg/kg -----------------------No Sludge/No Lime - pH 5.9 ND † 6.9 (1.8) TR † 32 (6.4) Sludge/No Lime - pH 4.6 12 (4.3) 36 (4.9) 81 (17) 1700 (360) Sludge/Lime - pH 5.8 7.6 (1.6) 31 (8.1) 20 (6.5) 630 (180) Sludge/Lime - pH 6.5 7.4 (1.6) 21 (3.3) 13 (5.4) 680 (130) Sludge/Lime - pH 6.9 5.8 (2.2) 23 (5.3) 9.7 (3.3) 570 (140) Amendment No Sludge/No Lime - pH 5.9 Sludge/No Lime - pH 4.6 Sludge/Lime - pH 5.8 Sludge/Lime - pH 6.5 Sludge/Lime - pH 6.9 Amendment No Sludge/No Lime - pH 5.9 Sludge/No Lime - pH 4.6 Sludge/Lime - pH 5.8 Sludge/Lime - pH 6.5 Sludge/Lime - pH 6.9 CABBAGE Cd Cu Ni Zn -------------------------- mg/kg -----------------------ND 2.1 (0.7) 2.7 (5.6) 20 (2.6) 8.2 (2.4) 9.0 (2.2) 34 (13) 900 (270) 1.5 (0.7) 8.6 (2.6) 27 (11) 200 (59) 1.2 (0.5) 6.9 (2.0) 19 (7.9) 160 (50) 1.6 (0.5) 9.5 (2.6) 22 (8.8) 190 (41) CORN STALK Cd Cu Ni Zn -------------------------- mg/kg -----------------------ND 1.7 (0.3) ND 18 (3.0) 4.5 (4.2) 4.1 (2.1) 3.6 (2.9) 230 (63) 7.9 (5.6) 9.6 (2.1) 37 (25) 480 (83) 3.7 (1.8) 7.5 (2.9) 9.4 (5.4) 440 (130) 4.3 (4.2) 6.7 (2.9) 22 (19) 430 (200) 45 46 Table 6 (con't): Mean (±S.D.) metal concentration (mg kg-1, dry wt.) in plant tissue grown in background (no sludge/no lime) and sludge-amended soils treated with lime to four different pH levels; pH 4.6, 5.8, 6.5 and 6.9. LETTUCE Amendment Cd Cu Ni Zn -------------------------- mg/kg -----------------------No Sludge/No Lime - pH 5.9 ND 10 (2.3) 5.9 (5.8) 41 (6.3) Sludge/No Lime - pH 4.6 3.2 (0.8) 9.5 (1.8) 13 (5.3) 360 (81) Sludge/Lime - pH 5.8 6.4 (2.9) 12 (2.6) 7.3 (2.4) 300 (85) Sludge/Lime - pH 6.5 2.9 (1.7) 11 (1.6) 3.8 (1.1) 170 (66) Sludge/Lime - pH 6.9 3.1 (1.1) 12 (2.6) 139 (33) 140 (33) Amendment No Sludge/No Lime - pH 5.9 Sludge/No Lime - pH 4.6 Sludge/Lime - pH 5.8 Sludge/Lime - pH 6.5 Sludge/Lime - pH 6.9 Amendment No Sludge/No Lime - pH 5.9 Sludge/No Lime - pH 4.6 Sludge/Lime - pH 5.8 Sludge/Lime - pH 6.5 Sludge/Lime - pH 6.9 TOMATO FRUIT Cd Cu Ni Zn -------------------------- mg/kg -----------------------ND 7.4 (1.3) 19 (17) 20 (1.9) 0.9 (0.3) 8.7 (4.2) 19 (13) 49 (9.4) 1.0 (0.7) 10 (2.4) 9.5 (3.8) 60 (12) TR 1.3 (3.3) 1.4 (3.7) 8.2 (21) TR TR TR TR POTATO TUBER PEELED Cd Cu Ni Zn -------------------------- mg/kg -----------------------ND 8.2 (2.2) 2.3 (5.1) 22 (4.1) 1.3 (0.3) 12 (3.5) 27 (32) 91 (30) TR 9.5 (1.7) 8.0 (5.0) 45 (17) TR 9.9 (1.7) 13 (22) 45 (8.4) TR 12 (1.6) 29 (43) 49 (6.8) 46 47 Table 6 (con't): Mean (±S.D.) metal concentration (mg kg-1, dry wt.) in plant tissue grown in background (no sludge/no lime) and sludge-amended soils treated with lime to four different pH levels; pH 4.6, 5.8, 6.5 and 6.9. POTATO PEEL Amendment Cd Cu Ni Zn -------------------------- mg/kg -----------------------No Sludge/No Lime - pH 5.9 TR 10 (1.7) 1.3 (0.3) 18 (2.8) Sludge/No Lime - pH 4.6 3.7 (1.2) 24 (9.0) 22 (8.3) 400 (170) Sludge/Lime - pH 5.8 2.3 (0.6) 20 (3.9) 10 (4.4) 130 (75) Sludge/Lime - pH 6.5 1.6 (0.3) 16 (3.3) 4.3 (2.3) 44 (11) Sludge/Lime - pH 6.9 1.4 (0.2) 20 (9.4) 3.6 (1.1) 42 (6.5) † ND and TR Values Cd Cu Ni Zn -------------------------- mg/kg -----------------------Nondetected (ND) ≤0.2 ≤0.3 ≤0.2 ≤0.2 Trace (TR) ≤0.5 ≤1.0 ≤0.8 ≤0.5 47 6. CONCLUSIONS Hypothesis 1: Extractability of the Cd, Cu, Ni, and Zn is a function of its free ion concentration (water soluble and exchangeable form). The most soluble and plant available soil fractions of Cd, Ni and Zn were the most significantly reduced by the liming treatment. Copper extractability was very low and soil Cu was present almost exclusively in the unavailable organically complexed or residual fraction even at the lowest soil pH level. The data for Cd, Ni and Zn appear to support the practice of liming soil to 6.0 or above when sludge is applied at a rate high enough to result in mineralization of excess nitrogen, nitrification, nitrate leaching and acidification of the soil. This study indicates trace metals in sludge-amended soils may remain plant available in acidic soils for several years after application. Soil pH will markedly alter the distribution of Cd, Ni and Zn among the plant available pools and should be considered carefully when assessing the fate of natural or applied trace metals. Using a sequential extraction technique to fractionate soil metals is critical when considering the fate of trace metals. This study shows the irrelavence of using only total metal concentrations to assess the relative mobility and plant availability of trace metals. Hypothesis 2: Plant availability of Cd, Cu, Ni, and Zn is a function of its free ion concentration. Cadmium, Cu, Ni and Zn remained plant available in a soil amended with a single high application of sewage sludge sixteen years earlier. Zinc was the most plant available sludge-borne metal. The ability for the absorbed Cu and Ni to move from vegetative parts into the fruit and tuber is substantially less than for Cd and Zn. This study also agrees with reported studies in which it has been shown that leafy green plant tissue have a higher trace metal 49 uptake rate than root or fruit tissue. General trends in the uptake of individual metals by the different crop tissue in this study (e.g., cabbage, lettuce and peeled potato tuber) suggest that crop uptake differences must be accounted for in food-chain risk assessment from land application of sewage sludge. In addition, sludge-borne metals generally do not cause serious toxicity to the majority of crops, even if large amounts of metal are added to the soil. 49 7. LITERATURE CITED Adriano, D.C., A.L. Page, A.A. Elseewi and A.C. Chang (1982) Cadmium availability to sudangrass grown on soils amended with sewage sludge and fly ash. J. Environ. Qual. 11: 197-203. Adriano, D.C. (1986) Trace Elements In The Terrestrial Environment. New York, NY, Springer-Verlag New York Inc. 501 pp. Alloway, B.J., M. Gregson, S.K. Gregson, R. Tanner and A. Till (1979) p. 545-548. In: Management and control of heavy metals in the environment. Int. Conf., London. Sept. 1979. CEP Consultants Ltd. Edinburgh. Beckett, P.H.T., R.D. Davis and P. Brindley (1979) The disposal of sewage sludge onto farmland: The scope of the problems of toxic elements. Water Pollut. Contr. 78: 419-445. Behel D., D.W. Nelson and L.E. Sommers (1983) Assessment of heavy metal equilibria in sewage sludge-treated soil. J. Environ. Qual. 12: 181-186. Berrow, M.L. and J.C. Burridge (1990) Persistence of metal residues in sewage sludge treated soils over seventeen years. Intern. J. Environ. Anal. Chem. 39: 173-177. Bidwell, A.M. and R.H. Dowdy (1987) Cadmium and Zn availability to corn following termination of sewage sludge application. J. Environ. Qual. 16: 438-442. Bingham, F.T., A.L. Page and J.R. Sims (1964) Retention of Cu and Zn by H-montmorillonite. Soil Sci. Soc. Am. Proc. 28: 351-354. Bingham, F.T., A.L. Page, R.J. Mahler and T.J. Ganje (1975) Growth and Cd accumulation of plants grown on a soil treated with Cd enriched sewage sludge. J. Environ. Qual. 4(2): 207-211. Bingham, F.T., A.L. Page, G.A. Mitchell and J.E. Strong (1979) Effects of liming an acid soil amended with sewage sludge enriched with Cd, Cu, 51 Ni and Zn on yield and Cd content of wheat grain. J. Environ. Qual. 8(2): 202-207. Boggess, S.F., S. Willavize and D.E. Koeppe (1978) Differential response of soybean varieties to soil Cd. Agron. J. 70: 756-760. Boswell, F.C. (1975) Municipal sewage sludge and selected element applications to soil: Effect on soil and fescue. J. Environ. Qual. 4: 267-273. Brady, N.C. (1990) Soil colloids: Their nature and practical significance. pp 177-212. In: The Nature and Properties of Soils. Macmillan Publishing Company, New York, N.Y. 621 pp. Calvet, R. and S. Bourgeois (1990) Some experiments on extraction of heavy metals present in soil. J. Environ. Anal. Chem. 39: 31-45. Campbell, J.H. and R.D. Evans (1987) Inorganic and organic ligand binding of Pb and Cd and resultant implications for bioavailability. Sci. Total Environ. 62: 219-227 Chaney, W.R., R.C. Strickland and R.J. Lamoreaux (1977) Phytotoxicity of Cd inhibited by lime. Plant and Soil. 47: 275-278. Chang, A.C., A.L. Page and J.E. Warneke (1987) Long-term sludge applications on Cd and Zn accumulation in swiss chard and radish. J. Environ. Qual. 16(3): 217-221. Chang, A.C., A.L. Page, J.E. Warneke, M.R. Risketo and T.E. Jones (1983) Accumulation of Cd and Zn in barley grown on sludge-treated soils: A long term field study. J. Environ. Qual. 12(3): 391-397. Corey, R.B., L.D. King, C. Lue-Hing, D.S. Fanning, J.J. Street and J.M. Walker (1987) Effect of sludge properties on accumulation of trace elements by crops. In: A.L. Page, T.J. Logan and J.A. Ryan (eds.) Land Application of Sludge: Food Chain Implications. Lewis Publishers, Chelsea, MI. Dijkshoorn, W., J.E.M. Lampe and L.W. Broekhoven (1981) Influence of soil pH on heavy metals in ryegrass from sludge-amended soils. Plant and Soil. 61: 277-284. 51 52 Dowdy, R.H. and W.E. Larson (1975) The availability of sludge-borne metals to various vegetable crops. J. Environ. Qual. 4(2): 278-282. Dudley, L.M., B.L. McNeal and J.E. Baham (1986) Time-dependent changes in soluble organics, Cu, Ni and Zn from sludge-amended soils. J. Environ. Qual. 15(2): 188-192. Ellis, B.G. and B.D. Knezek (1972) Adsorption reaction of micronutrients in soils. p. 59-78. In: J.J. Mortvedt, P.M. Giordano and W.J. Lindsay (eds.) Micronutrients in Agriculture. Soil Sci. Soc. Amer., Madison, WI. Emmerich, W.E., L.J. Lund, A.L. Page and A.C. Chang (1982) Solid phase forms of heavy metals in sewage sludge-treated soils. J. Environ. Qual. 11(2): 178-181. Essington, M.E. and S.V. Mattigod (1991) Trace element solid-phase associations in sewage sludge and sludge-amended soil. J. Environ. Qual. 55: 350-356. Farrah, H. and W.F. Pickering (1977) Influcence of clay-solute interactions on aqueous heavy metal ion levels. Water, Air, Soil Pollut. 8: 189197. Friesen, D.K., A.S.R. Juo and M.H. Miller (1980) Liming and limephosphorous-Zn interactions in two Nigerian Ultisols: I. Interactions in the soil. Soil Sci. Soc. Am. J. 44: 1221-1226. Fuller, W.H. and B.A. Alesii (1979) Behavior of municipal solid waste leachates: II. In soil. J. Environ. Sci. Health. A14(7): 559-592. Garland, T.R., D.A. Cataldo and R.E. Wildung (1981) J. Agric. Food Chem. 29: 915-920. Gerritse, R.G., R. Vriesema, J.S. Dalenberg and H.P. DeRoos (1982) Effect of sewage sludge on trace element mobility in soils. J. Environ. Qual. 11(3): 359-364. 52 53 Giordano, P.M., D.A. Mays and A.D. Behel Jr. (1979) Soil temperature effects on uptake of Cd and Zn by vegetable grown on sludgeamended soil. J. Environ. Qual. 8(2): 233-236. Giordano, P.M. and J.J. Mortvedt (1976) Nitrogen effects on mobility and plant uptake of heavy metals in sewage sludge applied to soil columns. J. Environ. Qual. 5(2): 165-168. Gupta, U.C., F.W. Calder and L.B. MacLeod (1971) Influence of added limestone and fertilizers upon the micronutrient content of forage tissue and soil. Plant and Soil. 35: 249-256. Haghiri, F.E. (1974) Plant uptake of Cd as influenced by CEC, organic matter, Zn and soil type. J. Environ. Qual. 3(2): 180-185. Harter, R.D. (1983) Effect of soil pH on adsorption of Pb, Cu, Zn and Ni. Soil Sci. Soc. Am. J. 47: 47-51. Hatch, D.J., L.H.P. Jones and R.G. Burau (1988) The effect of pH on the uptake of Cd by four plant species grown in flowing solution culture. Plant and Soil. 105: 121-126. Hatton, D. and W.F. Pickering (1980) The effect of pH on the retention of Cu, Pb, Zn and Cd by clay-humic acid mixtures. Water, Air, Soil Pollut. 14: 13-21. Hemphill, J.R., T.L. Jackson, L.W. Martin, G.L. Kiemnec, D. Hanson and V.V. Volk (1982) Sweet corn response to application of three sewage sludges. J. Environ. Qual. 11(2): 191-196. Henry, C.L. and R.B. Harrison (1992) Fate of trace metals in sewage sludge compost. p. 195-216. In: D.C. Adriano (ed) Biochemistry of Trace Metals. Lewis Publishers, CRC Press Inc., Boca Raton, FL. Hinesly, T.D. and L.G. Hansen (1984) Effects of Using Sewage Sludge on Agricultural and Disturbed Lands. U.S. EPA. 600/S2-83-113. PB 84117142. National Technical Information Service, Springfield, VA. Hinesly, T.D., R.L. Jones and E.L. Ziegler (1972) Effects on corn by application of heated anaerobically digested sludge. Compost Sci. 13: 26-30. 53 54 Hinesly, T.D., E.L. Ziegler and G.L. Barret (1979) Residual effects of irrigating corn with digested sewage sludge. J. Environ. Qual. 8(1): 35-38. Hodgson, J.F. (1963) Chemistry of the micronutrient elements in soils. Adv. Agron. 15: 119-159. Holtzclaw, K.M., D.A. Keech, A.L. Page, G. Sposito, T.J. Ganje and N.B. Ball (1978) Trace metal distributions among humic acid, the fulvic acid and precipitable fractions extracted with NaOH from sewage sludges. J. Environ. Qual. 7(1): 124-127. Iyengar, S.S., D.C. Martens and W.P. Miller. (1981) Distribution and plant availability of soil Zn fractions. Soil Sci. Soc. Am. J. 45: 735-739. Jung, J. and T.J. Logan (1992) Effects of sewage sludge Cd concentration on chemical extractability and plant uptake. J. Environ. Qual. 21: 7881. Kabata-Pendias, A. and H. Pendias (1984) Trace Elements in Soils and Plants. CRC Press Inc., Boca Raton, FL. 315 pp. Keeney, D.R. and R.E. Wildung (1977) Chemical properties of soil. p. 7594. In: L.F. Elliott and F.J. Stevenson (eds) Soils for Management of Organic Wastes and Waste Waters. Soil Sci. Soc. Amer., Madison, WI. King, L.D. (1988) Retention of metals by several soils of the southeastern United States. J. Environ. Qual. 17(2): 239-246. King, L.D. and W.R. Dunlop (1982) Application of sewage sludge to soils high in organic matter. J. Environ. Qual. 11(4): 608-616. Korcak, R.F. and D.S. Fanning (1985) Availability of applied heavy metals as a function of type of soil material and metal source. Soil Sci. 140(1): 23-34. Korte, N.E., J. Skopp, W.H. Fuller, E.E. Niebla and B.A. Alesii (1976) Trace element movement in soils: Influence of soil physical and chemical properties. Soil Sci. 122(6): 350-359. 54 55 Lagerwerff, J.V. (1972) Pb, Hg and Cd as contaminants. p. 593- 636. In: J.J. Mortvedt, P.M. Giordano and W.L. Lindsay (eds) Micronutrients in Agriculture. Soil Sci. Soc. of Am., Madison, WI. Lake, D.L., P.W.W. Kirk and J.N. Lester (1984) Fractionation, characterization, and speciation of heavy metals in sewage sludge and sludge-amended soils: A review. J. Environ. Qual. 13(2): 175-183. Latterell, J.J., R.H. Dowdy and G.E. Ham (1976) Sludge-borne metal uptake by soybeans as a function of soil cation exchange capacity. Commun. Soil Sci. and Plant Anal. 7: 465-476. Lindsay, W.L. (1979) Chemical equilibria in soils. John Wiley and Sons, New York, NY. 449 pp. Logan, T.J. and R.L. Chaney (1983) Metals. pp. 235-326. In: A.L. Page (ed.) Proceedings of the Workshop on Utilization of Municipal Wastewater and Sludge on Land. University of California, Riverside, CA. Logan, T.J. (1989) Sludge metal bioavailability. In: H.S. Muralidhara (ed.) Proc. Int. Symp. on Solid/Liquid Separations. Battelle Press, Columbus, OH. MacLean, A.J. and A.J. Dekker (1978) Availability of Zn, Cu and Ni to plants grown in sewage treated soils. Can. J. Soil Sci. 58: 381-389. Mahler, R.J., F.T. Bingham and A.L. Page (1978) Cadmium enriched sewage sludge application to acid and calcareous soils: Effect on yield and Cd uptake by lettcue and card. J. Environ. Qual. 7: 274-281. McBride, M.B. (1989) Reactions controlling heavy metal solubility in soils. pp. 1-56. In: B.A. Steward (ed.) Advances in Soil Science. SpringerVerlag, New York, N.Y. McCalla, T.M., J.R. Peterson and C. Lue-Hing (1977) Properties of agricultural and municipal wastes. pp. 11-41. In: L.F. Elliott and F.J. Stevenson (eds.) Soils for Management of Organic Wastes and Waste Waters. Soil Sci. Soc. Am., Madison, WI. 55 56 Parkinson, J.A. and S.E. Allen. (1975) A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun. Soil Sci. and Plant Anal. 6(1): 1-11. Petruzzelli, G., L. Lubrano and G. guidi (1981) The effect of sewage sludge and compost on the extractability of heavy metals from soil. Environ. Technol. Lett. 2: 449-456. Pepper, I.L., D.F. Bezdicek, A.S. Baker and J.M. Sims (1983) Silage corn uptake of sludge-applied Zn and Cd as affected by soil pH. J. Environ. Qual. 12: 270-275. Rappaport, B.D., D.C. Martins, R.B. Reneau and T.W. Simpson (1988) Metal availability in sludge-amended soils with elevated metal levels. J. Environ. Qual. 17(1): 42-47. Reddy, C.N. and W.H. Patrick, Jr. (1977) Effect of redox potential and pH on the uptake of Cd and Pb by rice plants. J. Environ. Qual. 6: 259-262. Schalscha, E.B., M. Morales, I. Ahumada, T. Schirado and P.F. Pratt (1980) Fractionation of Zn, Cu, Cr and Ni in watewaters, solids and in soil. Agrochimica. 24: 361-368. Schalscha, E.B., M. Morales, I. Vergara and A.C. Chang (1982) Chemical fractionation of heavy metals in wastewater affected soils. J. Water Pollut. Control Fed. 54: 175-180. Shuman, L.M. (1979) Zinc, Mn and Cu in soil fractions. Soil Sci. 127: 1017. Shuman, L.M. (1986) Effect of liming on the distribution of Mn, Cu, Fe and Zn among soil fractions. Soil Sci. Soc. Am. J. 50: 1236-1240. Silviera, D.J. and I.E. Sommers (1977) Extractability of Cu, Zn, Cd and Pb in soils incubated with sewage sludge. J. Environ. Qual. 6(1): 47-51. Sims, J.T. (1986) Soil pH effects on the distribution and plant availability of Mn, Cu and Zn. Soil Sci. Soc. Am. J. 50: 367-373. 56 57 Singh, B.R. and R.P. Narwal (1984) Plant availability of heavy metals in a sludge-treated soil: II. Metal extractability compared with plant metal uptake. J. Environ. Qual. 13(3): 344-348. Sposito, G.F., F.T. Bingham, S.S. Yadav and C.A. Inouye (1982) Trace metal complexation by fulvic acid extracted from sewage sludge: II. Development of chemical models. Soil Sci. Soc. Am. J. 46: 51-56. Soon, Y.K. and T.E. Bates (1982) Chemical pools of Cd, Ni and Zn in polluted soils and some preliminary indications of their availability to plants. J. Soil Sci. 33: 477-488. Street, J.J., B.R. Sabey and W.L. Lindsay (1978) Influence of pH, P, Cd, sewage sludge and incubation time on the solubility and plant uptake of Cd. J. Environ. Qual. 7(2): 286-290. Street, J.J., W.L. Lindsay and B.R. Sabey (1977) Solubility and plant uptake of Cd in soils amended with Cd and sewage sludge. J. Environ. Qual. 6: 72-77. SYSTAT (1990) Version 5.1. SYSTAT, Inc., Evanston, IL. Tessler, A., P.G.C. Campbell and M. Bisson (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 51(7): 844-851. U.S.D.A Soil Conservation Service (1979) Soil Survey of Pierce County Area, Washington. National Cooperative Soil Survey. 131 pp. U.S. EPA (1990) National Sewage Sludge Survey: Availability of Information and Data and Anticipated Impacts on Proposed Regulations: Proposed Rule 40 CFR Part 503. Federal Register 55:47210-47283. Verloo, M. and M. Eeckhout (1990) Metal species transformations in soils: An analytical approach. Intern. J. Environ. Anal. Chem. 39: 179-186. Vlamis, J., D.E. Williams, J.E. Corey, A.L. Page and T.J. Ganje (1985) Zinc and Cd uptake by barley in field plots fertilized seven years with urban and suburban sludge. Soil Sci. 139(1): 81-87. 57 58 Williams, D.E.; J. Vlamis, A.H. Purkite and J.E. Corey (1980) Trace element accumulation, movement and distribution in the soil profile from massive applications of sewage sludge. Soil Sci. 129(2): 119-131. 58 APPENDIX 1: PLANT YIELD DATA SAMPLE Total Above Ground Wet Wt (kg) Edible Wet Wt.* (kg) Plant Ht (cm) LETTUCE Mean SD Mean SD Mean SD Background-pH 5.9 0.13 0.08 0.13 0.08 19.1 5.8 Sludge/No lime-pH 4.6 0.07 0.04 0.03 0.04 8.7 4.0 Sludge/Lime-pH 5.8 0.39 0.11 0.28 0.09 25.8 1.7 Sludge/Lime-pH 6.5 0.48 0.14 0.35 0.11 25.1 2.1 Sludge/Lime-pH 6.9 0.40 0.13 0.27 0.11 24.3 2.9 * Weight minus outer wrapper leaves SAMPLE Total Above Ground Wet Wt (kg) Edible Wet Wt.* (kg) Plant Ht (cm) CABBAGE Mean SD Mean SD Mean SD Background-pH 5.9 0.51 0.21 0.13 0.08 21.8 3.5 Sludge/No lime-pH 4.6 0.02 0.02 0.02 0.02 11.5 3.8 Sludge/Lime-pH 5.8 0.84 0.40 0.23 0.13 27.4 2.2 Sludge/Lime-pH 6.5 0.88 0.33 0.24 0.14 25.6 2.6 Sludge/Lime-pH 6.9 0.56 0.21 0.13 0.07 22.6 2.2 * 1/2 of head minus outer wrapper leaves SAMPLE Total Above Ground Wet Wt (g) Plant Ht (cm) BUSH BEAN Mean SD Mean SD Background-pH 5.9 28.00 7.13 --- --- Sludge/No lime-pH 4.6 0.74 0.15 5.1 0.3 Sludge/Lime-pH 5.8 25.83 11.47 28.8 6.6 Sludge/Lime-pH 6.5 23.35 6.13 26.2 4.1 Sludge/Lime-pH 6.9 25.28 11.63 27.6 3.8 60 60 61 APPENDIX 1: Plant Yield Data (con't) SAMPLE Individual Tuber Wt.* Unpeeled Tuber Wet Wt (g) POTATO TUBER Mean SD Background-pH 5.9 82.78 34.48 Sludge/No lime-pH 4.6 43.70 37.12 Sludge/Lime-pH 5.8 146.89 73.84 Sludge/Lime-pH 6.5 154.66 57.03 Sludge/Lime-pH 6.9 165.81 64.58 *Total above ground plant wet wt. was not measured at time of harvest. SAMPLE Individual Tuber Wt. Unpeeled Tuber Wet Wt (g) Tuber Length (cm) CARROT TUBER Mean SD Mean SD Background-pH 5.9 27.99 10.41 28 12 Sludge/No lime-pH 4.6 Non Non producing producing Sludge/Lime-pH 5.8 25.78 16.55 23 9 Sludge/Lime-pH 6.5 20.79 10.37 23 9 Sludge/Lime-pH 6.9 19.09 6.43 23 9 SAMPLE Total Above Ground Wet Wt (kg) above 2nd leaf Plant Ht (cm) CORN STALK Mean SD Mean SD Background-pH 5.9 0.25 0.04 113.5 9.7 Sludge/No lime-pH 4.6 0.31 0.08 103.3 13.9 Sludge/Lime-pH 5.8 0.56 0.09 129.8 20.7 Sludge/Lime-pH 6.5 0.41 0.13 113.3 8.4 Sludge/Lime-pH 6.9 0.43 0.12 122.5 21.8 61 62 APPENDIX 1: Plant Yield Data (con't) SAMPLE TOMATO Total Above Ground Total Fruit Yield Wet Wt (g) Wet Wt (g) Plant Ht (cm) Mean SD Mean SD Mean SD Background-pH 5.9 745 184 345 133 110 12 Sludge/No lime-pH 4.6 138 85 57 44 56 10 Sludge/Lime-pH 5.8 2171 1839 718 752 85 15 Sludge/Lime-pH 6.5 1470 541 900 341 115 12 Sludge/Lime-pH 6.9 1864 576 1143 256 119 11 62 63 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS * Description of Type coding. An entry of 9999 signifies no sample: BL = Bean Leaf CBL = Background pH 5.9 (no sludge/no lime) Bean Leaf S5.0BL = Sludge/No lime pH 4.6 Bean Leaf S5.5BL = Sludge/Lime pH 5.8 Bean Leaf S6.0BL = Sludge/Lime pH 6.5 Bean Leaf S6.5BL = Sludge/Lime pH 6.9 Bean Leaf CB = Cabbage CCB = Background pH 5.9 (no sludge/no lime) Cabbage S5.0CB = Sludge/No lime pH 4.6 Cabbage S5.5CB = Sludge/Lime pH 5.8 Cabbage S6.0CB = Sludge/Lime pH 6.5 Cabbage S6.5CB = Sludge/Lime pH 6.9 Cabbage CP = Carrot Peel CCP = Background pH 5.9 (no sludge/no lime) Carrot Peel S5.0CP = Sludge/No lime pH 4.6 Carrot Peel S5.5CP= Sludge/Lime pH 5.8 Carrot Peel S6.0CP = Sludge/Lime pH 6.5 Carrot Peel S6.5CP = Sludge/Lime pH 6.9 Carrot Peel CT = Peeled Carrot Tuber CCT = Background pH 5.9 (no sludge/no lime) Peeled Carrot Tuber S5.0CT = Sludge/No lime pH 4.6 Peeled Carrot Tuber S5.5CT= Sludge/Lime pH 5.8 Peeled Carrot Tuber S6.0CT = Sludge/Lime pH 6.5 Peeled Carrot Tuber S6.5CT = Sludge/Lime pH 6.9 Peeled Carrot Tuber 63 64 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS (CON'T) PP = Potato Peel CPP = Background pH 5.9 (no sludge/no lime) Potato Peel S5.0PP = Sludge/No lime pH 4.6 Potato Peel S5.5PP= Sludge/Lime pH 5.8 Potato Peel S6.0PP = Sludge/Lime pH 6.5 Potato Peel S6.5PP = Sludge/Lime pH 6.9 Potato Peel PT = Peeled Potato Tuber CPT = Background pH 5.9 (no sludge/no lime) Peeled Potato Tuber S5.0PT = Sludge/No lime pH 4.6 Peeled Potato Tuber S5.5PT= Sludge/Lime pH 5.8 Peeled Potato Tuber S6.0PT = Sludge/Lime pH 6.5 Peeled Potato Tuber S6.5PT = Sludge/Lime pH 6.9 Peeled Potato Tuber T = Tomato Fruit CT = Background pH 5.9 (no sludge/no lime) Tomato Fruit S5.0T = Sludge/No lime pH 4.6 Tomato Fruit S5.5T= Sludge/Lime pH 5.8 Tomato Fruit S6.0T = Sludge/Lime pH 6.5 Tomato Fruit S6.5T = Sludge/Lime pH 6.9 Tomato Fruit L = Lettuce CL = Background pH 5.9 (no sludge/no lime) Lettuce S5.0L = Sludge/No lime pH 4.6 Lettuce S5.5L = Sludge/Lime pH 5.8 Lettuce S6.0L = Sludge/Lime pH 6.5 Lettuce S6.5L = Sludge/Lime pH 6.9 Lettuce 64 65 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS (CON'T) 65 66 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS (CON'T) 66 67 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS (CON'T) 67 68 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS (CON'T) 68 69 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS (CON'T) 69 70 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS (CON'T) 70 71 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS (CON'T) 71 72 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS (CON'T) 72 73 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS (CON'T) 73 74 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS (CON'T) 74 75 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS (CON'T) 75 76 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS (CON'T) 76 77 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS (CON'T) 77 78 APPENDIX 2: COMPLETE PLANT DATA ANALYSIS (CON'T) 78