Chapter 4.1 Notes
advertisement
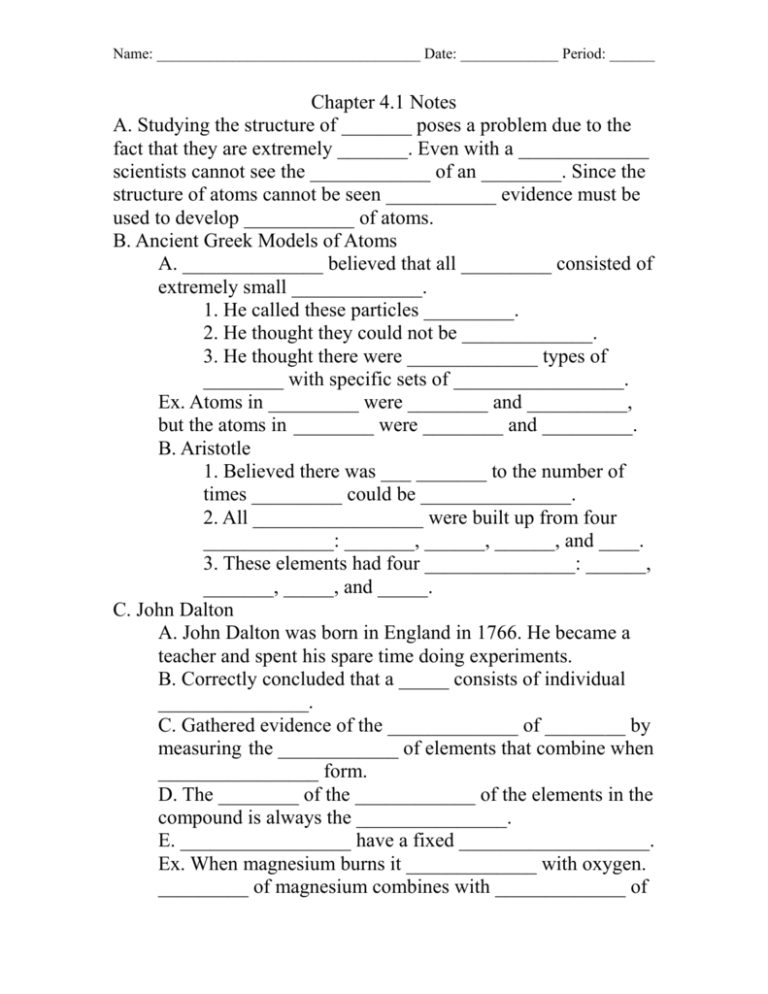
Name: ___________________________________ Date: _____________ Period: ______ Chapter 4.1 Notes A. Studying the structure of _______ poses a problem due to the fact that they are extremely _______. Even with a _____________ scientists cannot see the ____________ of an ________. Since the structure of atoms cannot be seen ___________ evidence must be used to develop ___________ of atoms. B. Ancient Greek Models of Atoms A. ______________ believed that all _________ consisted of extremely small _____________. 1. He called these particles _________. 2. He thought they could not be _____________. 3. He thought there were _____________ types of ________ with specific sets of _________________. Ex. Atoms in _________ were ________ and __________, but the atoms in ________ were ________ and _________. B. Aristotle 1. Believed there was ___ _______ to the number of times _________ could be _______________. 2. All _________________ were built up from four _____________: _______, ______, ______, and ____. 3. These elements had four _______________: ______, _______, _____, and _____. C. John Dalton A. John Dalton was born in England in 1766. He became a teacher and spent his spare time doing experiments. B. Correctly concluded that a _____ consists of individual _______________. C. Gathered evidence of the _____________ of ________ by measuring the ____________ of elements that combine when ________________ form. D. The ________ of the ____________ of the elements in the compound is always the _______________. E. _________________ have a fixed ___________________. Ex. When magnesium burns it _____________ with oxygen. _________ of magnesium combines with _____________ of Name: ___________________________________ Date: _____________ Period: ______ oxygen. ______of magnesium combines with _______ of oxygen. D. Dalton’s Atomic Theory A. ________ is made up of individual _____________ called ______________, which cannot be ________________. 1. All _____________ are composed of ________. 2. All ______ of the ____ element have the same ____, and atoms of ________ elements have different _____. 3. _________ contain atoms of _____ than one ______. 4. In a particular _________, atoms of different ____________ always combine in the _________ way. B. While this theory was _____ perfect it was not _________ but revised to __________ for new ________________. Pic: Dalton represented __________ as solid ________. Each type of _______ is represented by _____, _______ spheres with a different _________. C. What did Dalton notice that all compounds have in common? ______________________________________________________ ____________________________________________________ E. Joseph John Thomson (1856-1940), J.J. Thomson, used an _________________ current to learn more about _________. A. The device used to create an electric current was a ______ ____ with most of the ____ vacuumed out sealed with a ________ disk on each end. When the electric current is turned on one ________ becomes ______________ charged while the other disk becomes ________________ charged. This created a glowing ________ in the glass tube. B. Thomson hypothesized that the _______ was a stream of ________ particles that interacted with the ____ in the tube and caused the air to _________. C. Thomson found, in an experiment, that the _________ of charged ___________ was attracted to a ________________ charged plate. D. He __________________ that the particles came from ___________ atoms. Name: ___________________________________ Date: _____________ Period: ______ E. Thomson’s experiments provided the ______ evidence that ________ are made of even _____________ particles. F. This changed how scientists thought about atoms. G. Thomson revised ____________ model to account for these _______________ particles. F. Thomson’s Model A. An atom is _____________, meaning it has neither a ____________ nor a positive charge. However, Thomson knew from his experiments that _________ contained negatively charged _____________. B. How can an atom contain negative particles and still be neutral? C. Plum Pudding Model (Chocolate Chip Ice Cream) 1. In Thomson’s model the ___________ charges were ______ scattered throughout an _____ filled with a _____________ charged _________ of matter. 2. Think of _____________ ________ as negative particles and the ____ ________ as a positively charged mass of ________. With the chocolate chips spread _______ throughout the ice cream the ________ of the chocolate chips and ice cream ___________ each other out and create an overall ___________ charge. D. What evidence did Thompson’s experiments provide? ________________________________________________. G. Ernest Rutherford (1871-1937) A. Surprised by _____________ results that lead to important _________________. B. Discovered that _____________ emits fast moving ________________ that have a _____________ charge. He named these particles ___________ particles. C. Rutherford’s experiment consisted of sending a ______ of alpha particles through a thin sheet of ______ ___________. D. He hypothesized, based on ____________ model, that the Name: ___________________________________ Date: _____________ Period: ______ _____ and ______ of the alpha particles traveling through the gold foil would be too ________ to change the _____ of the particles. The particles would travel ______ through the gold foil and ________ a screen that would ______ ___ when hit. E. Some of the ____________ that hit the gold foil were ____________. He found that his hypothesis was _______. F. This led Rutherford to believe that the ___________ charge of an _________ is not __________ spread throughout the atom. G. The ____________ charge is _______________ in a very small, central area called the _______________. H. The particles that were ________________ came close to a ___________, while the ones that traveled ___________ through did not. I. Rutherford’s __________ showed that all of the ________ charged particles of an _______ are concentrated in its ____________ not spread ______ out like Thomson’s model. Scientist Evidence Model Ratio of masses Deflected Beam of charged particles Rutherford Nucleus Demonstration: Atomic Theory Alcohol Swab 1. Where does the alcohol go? __________________________ ___________________________________________________ 2. Does it leave the paper all at once in one big chunk? _______ ___________________________________________________ 3. How does this observation show that the alcohol must actually be made of individual particles? ________________ ___________________________________________________ ___________________________________________________









