249115_2_Sep_Lab_2015
advertisement

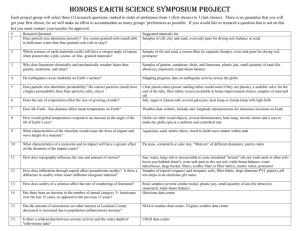
Separation Lab Purpose: In nature matter is mostly in mixtures. In this lab you will be given a mixture of two pure substances. Your goal will be to separate the two substances from each other and then obtain the mass of each pure substance. Since the amounts of each pure substance are known by the instructor, you will be able to determine the success of your lab based on how close you are to the actual amount of each pure substance. Materials: Vial containing a mixture of sand and salt , hotplate, 250 mL beaker, funnel, filter paper, ring stand, stirring rod Pre-lab: 1. After getting into a group of four, discuss how you could best separate the mixture and obtain the mass of the sand and the salt. Make a step-by-step procedure in your lab book and sure to include each mass you much obtain. When done, have one group member present their procedure to the instructor. 2. Then obtain the actual procedure and attach to your lab book. 3. Prepare a data table that includes all masses you must obtain. Separation Lab Purpose: In nature matter is mostly in mixtures. In this lab you will be given a mixture of two pure substances. Your goal will be to separate the two substances from each other and then obtain the mass of each pure substance. Since the amounts of each pure substance are known by the instructor, you will be able to determine the success of your lab based on how close you are to the actual amount of each pure substance. Materials: Vial containing a mixture of sand and salt , hotplate, 250 mL beaker, funnel, filter paper, ring stand, stirring rod Pre-lab: 1. After getting into a group of four, discuss how you could best separate the mixture and obtain the mass of the sand and the salt. Make a step-by-step procedure in your lab book and sure to include each mass you much obtain. When done, have one group member present their procedure to the instructor. 2. Then obtain the actual procedure and attach to your lab book. 3. Prepare a data table that includes all masses you must obtain. Procedure: Day 1. 1. Obtain sand/salt mixture. Record vial number. 2. Add about 30 mL of DI water to vial. Stir until salt is dissolved. 3. Mass dry filter paper. Write initials and period on top of paper 4. Mass dry 250 mL or 125 mL beaker. Write initials and period on top of beaker. 5. Place filter paper in funnel, place beaker under funnel and pour water through filter paper. Make sure you do this as shown by instructor. 6. Wash all sand onto filter paper. Rinse several times with DI water. 7. Carefully fold filter paper, ensuring that initials are visible. Place in oven overnight. 8. Place beaker on hotplate and boil down liquid until salt almost appears. 9. Place beaker in oven overnight. Day 2: Mass filter paper containing sand and beaker containing salt. Place back in oven for 15 minutes, cool and remass. If masses are within 1% then samples are dry. Procedure: Day 1. 1. Obtain sand/salt mixture. Record vial number. 2. Add about 30 mL of DI water to vial. Stir until salt is dissolved. 3. Mass dry filter paper. Write initials and period on top of paper 4. Mass dry 250 mL or 125 mL beaker. Write initials and period on top of beaker. 5. Place filter paper in funnel, place beaker under funnel and pour water through filter paper. Make sure you do this as shown by instructor. 6. Wash all sand onto filter paper. Rinse several times with DI water. 7. Carefully fold filter paper, ensuring that initials are visible. Place in oven overnight. 8. Place beaker on hotplate and boil down liquid until salt almost appears. 9. Place beaker in oven overnight. Day 2: Mass filter paper containing sand and beaker containing salt. Place back in oven for 15 minutes, cool and remass. If masses are within 1% then samples are dry. Lab Report: Separation. Follow the handout on lab reports for all sections. Post Lab Data Analysis 1. Analyze the data: What was the mass of your sand and your salt? How do they compare to each other? 2. Make a conclusion: Indicate whether the sand and salt were separated successfully by comparing to the actual numbers given to you by the instructor. Also, mention the actual % error for the sand and for the salt. 3. Discuss Errors: Focus on your % error. Give specific reasons why your sand value was higher or lower than the actual. Give specific reasons why the salt value was higher or lower than the actual. 4. Alex was separating a mixture of sand and salt. After collecting the sand on filter paper Alex decided not to wash the sand with water to save time. How will this change the amount of sand and the amount of salt he reports. (Be specific) Lab Report: Separation. Follow the handout on lab reports for all sections. Post Lab Data Analysis 1. Analyze the data: What was the mass of your sand and your salt? How do they compare to each other? 2. Make a conclusion: Indicate whether the sand and salt were separated successfully by comparing to the actual numbers given to you by the instructor. Also, mention the actual % error for the sand and for the salt. 3. Discuss Errors: Focus on your % error. Give specific reasons why your sand value was higher or lower than the actual. Give specific reasons why the salt value was higher or lower than the actual. 4. Alex was separating a mixture of sand and salt. After collecting the sand on filter paper Alex decided not to wash the sand with water to save time. How will this change the amount of sand and the amount of salt he reports. (Be specific) Lab Report: Separation Makeup Follow the handout on lab reports for all sections. Post Lab Data Analysis 1. Analyze the data: What was the mass of your sand and your salt? How do they compare to each other? 2. Make a conclusion: Indicate whether the sand and salt were separated successfully by comparing to the actual numbers given to you by the instructor. Also, mention the actual % error for the sand and for the salt. 3. Discuss Errors: Focus on your % error. Give specific reasons why your sand value was higher or lower than the actual. Give specific reasons why the salt value was higher or lower than the actual. 4. Alex was separating a mixture of sand and salt. After collecting the sand on filter paper Alex decided not to wash the sand with water to save time. How will this change the amount of sand and the amount of salt he reports. (Be specific) Data for makeup Item Beaker Filter paper Beaker w/salt Filter paper/sand Mass(g) 98.234 1.845 99.391 2.456