Chemistry-notes-ch-13
advertisement

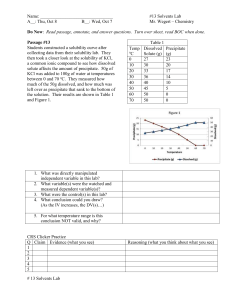
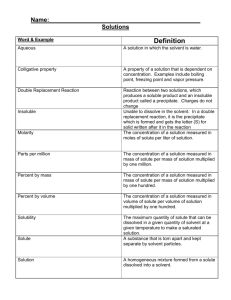
Chemistry Notes: Chapter 13 Solutions Soluble—means that a substance is capable of being dissolved to form a solution such as sugar or salt in water. Solution—a homogenous mixture of two or more substances in a single phase. A solution has the same composition and properties throughout. Solutions are always homogenous. Solutions have two components: Solute: substance that is being dissolved. (usually the substance of lesser quantity) Solvent—the dissolving medium. There are different types of solutions: See table 13.1 Many alloys such as brass and sterling silver are solid solutions. Brass is made of zinc and copper and sterling silver is made of silver and copper. By varying the amts of each substance, many desirable properties can be obtained. Pure gold (24 K) is much too soft to be used in jewelry so it is mixed with silver to increase the strength and hardness. Suspensions—A mixture where the particles in the solvent are so large that they settle out if left alone. Muddy river water is a suspension. Suspensions are always heterogeneous. Colloids—formed from a mixture of particles that are intermediate in size between solutions and suspensions. Colloid particles are large enough to scatter light but small enough so they don’t settle out. The colloid particles make up the dispersed phase and water is the dispersing medium. See table 13-2 for different classes of colloids. Tyndall Effect—the scattering of light by colloidal particles in a transparent medium. (Headlights reflecting off of particles in fog.) All substances that dissolve in water are classified by whether or not they form ions in solution. Electrolytes—substances that conduct a current when dissolved in water. In order to conduct a current, the solution must form ions when dissolved. Examples—all ionic substances, such as salts, that dissolve in water. Also, some polar molecules such as HCl because the polarity of the water pulls them apart and forms ions. Nonelectrolytes—substances that do not conduct an electric current when dissolved in water. They do not form ions in solution. All nonpolar molecular substances that dissolve in water such as sugar. 13-2 Factors that affect the rate of dissolution. 1. Increasing the surface area of the solute. a. grind it up 2. Stirring the solution. 3. Heating the solvent usually increases the dissolving process but not always. How much of a solute dissolves in a certain solvent depends on three things: 1 1. the nature of the solute 2. the nature of the solvent 3. the temperature (read p. 402, 3rd paragraph Solution Equilibrium—physical state in which the opposing processes of dissolution and crystallization of a solute occur at equal rates. Saturated Solution-one that contains the maximum amount of dissolved solute. Unsaturated solution—one that does not contain the maximum amount of dissolved solute at the given conditions. Supersaturated solution—one that contains more dissolved solute than a saturated solution contains under the same conditions. Solubility—amount of a substance required to form a saturated solution with a specific amount of solvent at a specified temperature. Must be determined experimentally and vary greatly. Tables give solubilities in such units as g/100 g of solvent or g/100 ml of solvent. Temperature must be specified and if the solute is a gas, pressure must also be specified. Interactions between solute and solvent particles. Good rule of thumb: Likes dissolve likes. Polar substance will dissolve other polar substances. Nonpolar substances will dissolve other nonpolar substances. Hydrolysis—when water is the solvent. Because of water’s polar properties, water dissolves ionic substances. The charged ends of water molecules attract and surround the ions in the solution. The ions are said to be “hydrated”. When crystallized, some ionic substances incorporate water molecules into their crystals. These substances, such as CuSO4 5H2O are known as hydrates. Ionic substances are generally not soluble in nonpolar solvents such as CCl4 because the nonpolar molecules do not attract the ions of the crystal to pull away. Miscible—liquids that dissolve freely in one another in any proportion such as ethanol and water. Immiscible—liquids solutes and solvents that are not soluble in each other such as oil and water. Oil will, however, dissolve in other nonpolar solvents such as CCl4. Ethanol is unique because part of it is polar and part of it is nonpolar so it will dissolve both polar or ionic substances and nonpolar substances. Pressure has very little effect on the solubilities of liquids or solids in liquids. However, pressure greatly affects how much gas can dissolve in a liquid. Increasing pressure increases the solubility of gases in liquids such as CO2 in pop. 2 Henry’s Law: The solubility of a gas in a liquid is directly proportional to the partial pressure of that gas on the surface of the liquid. Effervescence—the rapid escape of a gas from the liquid in which it is dissolved. (Fizzing of pop when you open the can) Increasing temperature usually decreases the solubility of a gas in a liquid. (lakes in the summer) Increasing temperature usually increases the solubility of solids in liquids but not always. Sometimes increasing temperature decreases dissolving. Also, the effects are not uniform—see figure 13-15. A solute particle that is surrounded by solvent molecules is said to be “solvated”. Heat of solution—the net amount of heat energy absorbed or releases when a specific amount of solute dissolves in a solvent. + heats of solution—heat is absorbed and the process is endothermic. - heats of solution—heat is given off and the process is exothermic. - See table 13-5 Concentrations of Solutions—measures the amount of solute in a given amount of solvent or solution. 1. Molarity—number of moles of solute/ liter of solution. 2. Molality—number of moles of solute/Kg of solvent. (sample problems 414-418) 3