study guide answers ch. 7 Unit 1 Test
advertisement

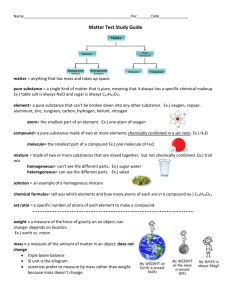
Name ____________________ Period ___________ Study Guide for Unit 1 Test 8th Grade Science METRIC Based on what number? 10 Metric Tool What does it measure? Triple Beam Balance- mass Graduated Cylinder- volume of liquid or displacement for volume of a solid; Metric Units grams mL ( liquid ) or Cm3 for an irregular solid Metric Rulerdistance or length cm Thermometertemperature degrees Celsius KHDbdch means??? Kilo – 1000; Hecto- 100; Deka – 10 ; base = 1 deci – .1; centi -.01; milli- .001 *****BE ABLE TO DO A CONVERSION. EX: 25.2 km = ? dm ? 250, 000 dm Definition Units Mass- amount of matter in an object grams Volume-amount of space an object or substance takes up mL ( liquid ) or Cm3 for a solid Volume of a liquid : What tool do you use? Graduated cylinder Volume of a regular solid- What tool do you use? Meter stick/m. ruler -Formula: L x W x H Volume of an irregular solid: Tool ? Graduated cylinder or overflow can unit Cm3 ( remember: 1 mL = 1cm3 ) Calculate using pictures: 32 mL ; then 38 mL = 6 mL difference = 6 Cm3 MATTER (define) : anything that has mass and volume; can be pure substances ( elements or compounds) or mixtures ( not pure, but can have pure substances in them ) Pure Substances- either elements, molecules or compounds. They are chemically combined in molecule and compound form. They have a specific ratio of elements chemically combined to be pure. In element form, they are only made up of 1 kind of atom. Element- simplest pure substance; made up of only 1 kind of atom. Compound-made up of 2 or more different atoms chemically combined in a specific ratio. Ex: CO2 has 1 Carbon atom chemically bonded to 2 Oxygen atoms. Mixtures (define and give an example) Homogenous2 or more substances physically combined so they all “look” the same, but they are not! They are evenly mixed, and particles are dissolved ! Difficult to separate, but can be through evaporation, filtering, etc. Ex: brownie batter; milk; vanilla ice cream( has milk, sugar, vanilla flavoring and other stuff mixed physically, but it all looks the same ); tea; coffee; lemonade Heterogeneous2 or more substances physically combined; all looks different; large particles; can easily be separated. Unevenly mixed, and particles are NOT dissolved. Ex: pizza; hamburger with tomato and bun; oreo cookie; chocolate chip cookie; Solution- has 2 parts; solute and solvent; homogeneous mixture; particles are dissolved. All looks the same. Solute is part that dissolves; Solvent dissolves the solute. Ex: salt is solute and water is solvent. Suspension-Heterogeneous mixture ; cloudy; has to be shaken to be mixed; settles out while standing. Ex: oil and vinegar; snow globe; dust in air; pond water and rocks PROPERTIES of matter (define and list) Physical – observable and measurable; color, size, shape, texture; melting and freezing pts, boiling pts; malleable; ductile; viscosity; density, mass, weight, volume , phase or state of matter. Chemical – characteristics that can cause change in substance. Ex: flammability; ability to rust, ability to react with water, acid or other substances; CHANGES OF MATTER: (define): Physical – change in form, but nothing new is created. Ex: cutting, folding, melting, freezing, subliming, boiling, evaporating, painting a chair Chemical -change in substance to become something new. Ex: fizzing, bubbling, reacting with any substance; rusting; combusting, burning, change in color that is new; cooking ; creating a gas; change in pH. PLS. USE ALL YOUR WORKSHEETS FOR THE PHYSICAL/CHEMICAL PROPERTIES/CHANGES.