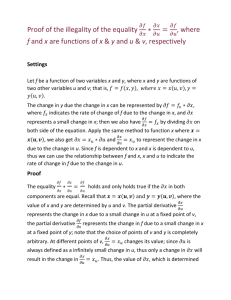
Identification of an unknown carbonyl
advertisement

Identification of an unknown carbonyl Practicum You will be given an unknown aldehyde or ketone. You will be given IR and nmr spectra, and you must prepare two solid derivatives. In the report, identify the unknown and compare the experimental values with the ones given in the table of possible compounds. Be sure to include balanced equations for the preparations of the derivatives, as well as appropriate mechanisms. Do not weigh derivatives or calculate % yield. Prepare the 2,4-dinitrophenylhydrazone derivative of your unknown. The reagent is already prepared. Mix 5 drops (0.25 mL) of your unknown in 10 mL of 95% ethanol. To this solution, add 5 mL of the 2,4-DNPH reagent. Shake the mixture vigorously. If a precipitate does not form immediately, let it stand for 15 minutes. Suction filter the solid derivative and recrystallize from 95% ethanol. After air drying, obtain the mp. Make an additional derivative, the semicarbazone, according to the directions on the following page and obtain the mp. Do a simple distillation to measure the boiling point of your unknown carbonyl compound. Label all definable IR and NMR peaks on your spectra. Answer the following questions: 1) An unknown organic compound (b. 212-216 oC) gives a positive 2,4-DNPH test and is positive with Tollen's reagent. A semicarbazone derivative is made that melts at 228-232 oC. What is the identity of the unknown? What would you do next? 2) Predict the products of the reaction of the following with silver nitrate in ammonium hydroxide: cylcohexanone formaldehyde acetone acetophenone butyraldehyde 3) In the reaction of an aldehyde or ketone with derivatives of ammonia, the reaction can be catalyzed by sulfuric acid. However, it is important that the pH not be too low since the reaction will slow down at very high acid concentrations. Explain. 4) Ketones do not oxidize readily. However, cyclohexanone will react with powerful oxidizing agents at high heat to adipic acid (HO2C-(CH2)4-CO2H). The reaction is not really one of the ketone, but the enol. Write equations to show how this is possible. Oxime Derivative Preparation Disolve 0.5 gm. hydroxylamine hydrochloride in 5 mL. of water and 3 mL. of 3M NaOH. Add 0.5 gm./mL of your unknown to the above solution. Warm the mixture in a boiling water bath for 10 minutes. Cool in an ice bath and collect the crystals using vacuum filtration. Dry overnight and determine the compound’s melting range. Semicarbazone Derivative Preparation Disolve 0.5 gm. semicarbazide hydrochloride and 0.8 gm. of Na(OAc) in 5 mL. of water. Add 0.5 gm. of your compound, stopper and shake the solution vigorously. Warm the mixture in a boiling water bath for 10 minutes. Remove the beaker from the heat source and allow the solution to cool to cool to room temperature in the beaker. Collect the crystals using vacuum filtration.