Experiment 6
advertisement

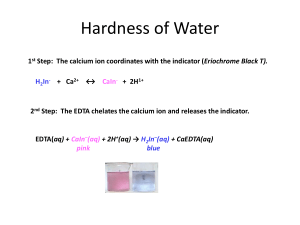
Experiment six: EDTA titration of the Hardness of Water Andrew Mullenax Purpose: The purpose of this experiment is to prepare a procedure to determine the hardness of water. Procedure: 1. Calibrate a pH meter with buffer solutions until the pH of 6M ammonia buffer can be determined. 2. Add concentrated HCl to the ammonia buffer until a pH of 10 is reached. 3. Prepare a 0.01M EDTA solution by weighing approximately 0.9360g EDTA and add 4mL of the 6M ammonia buffer in a 250mL volumetric flask and fill to the mark. 4. Prepare a calcium chloride solution by mixing approximately 0.5000g CaCO3 with 100mL of 0.1M HCl. 5. Place 3mL of your CaCl2 solution in a 250mL Erlenmeyer flask with 5mL ammonia buffer as well as a small amount of calmagite indicator. 6. Titrate the solution with your EDTA solution until the violet turns to a blue. Repeat for three good trials. 7. Pipet 50mL of tap water into a 250mL Erlenmeyer flask. Add 3mL of ammonia buffer and a small amount of calmagite indicator. 8. Titrate this solution with your EDTA solution. Repeat for three good trials. 9. Crush and weigh a tums tablet. Add it to a 100mL volumetric flask. Add 5mL ammonia buffer then dilute to the mark with 0.1M HCl. 10. Place 5mL of this solution in a 250mL Erlenmeyer flask. Add 2mL of ammonia buffer and a small amount of calmagite indicator. 11. Titrate until the solution turns from a pink to a blue color. Data: Standardization of EDTA Mass CaCO3: 0.5000g Mass EDTA: 0.9375g pH of Ammonia Buffer: 9.65 Volume EDTA Added(mL) Concentration of EDTA Trial 1 Trial 2 Trial 3 Average 16.12 14.88 15.10 15.37 .00930 .01007 .00992 0.00976 Titration of Tap Water Volume EDTA added(mL) Concentration of Ca2+ Hardness (ppm) Trial 1 Trial 2 Trial 3 Average 5.71 5.01 5.50 5.41 .00101 .00089 .00098 0.00096 40.51 35.69 39.30 38.50 Trial 1 Trial 2 Trial 3 Average 15.9 14.5 14.9 15.1 7.21% 6.58% 6.76% 6.85% Titration of Antacid Tablet Mass of tablet: 1.7258g Volume EDTA added(mL) % Ca2+ in Sample Calculations: Mass EDTA needed: 372.24g/mol × 372.24g/mol × 0.010 mol × 0.25 L 1L 0.010 mol × 0.25 L = 0.9306 g EDTA 1L Mass Calcium Carbonate needed: Molarity of CaCl2 × L CaCl2 used × 0.1 M CaCl2 × 0.100L CaCl2 × 1 mol CaCO3 molar mass CaCO3 × 1 mol CaCl2 1 mol CaCO3 1 mol CaCO3 100.09 CaCO3 × = 1.0 g 1 mol CaCl2 1 mol CaCO3 Molarity EDTA: mass CaCO3 × 0.500 g CaCO3 × 1 mol CaCO3 1 mol 1 × × 0.003L CaCl2 × 100.1g CaCO3 0.1 L L of EDTA 1 mol CaCO3 1 mol 1 × × 0.003L CaCl2 × = 0.0093M EDTA 100.09 g CaCO3 0.1 L 0.01612mL of EDTA Ca concentration in tap water: L of EDTA added × Molarity EDTA × 0.00571L × 0.0098M × 1 mol Ca 1 × 1 mol EDTA 0.055 L 1 mol Ca 1 × = 0.00102 M 1 mol EDTA 0.055 L Calcium Hardness of Tapwater: Ca Molarity × 0.00102 M × mass Ca 1000 mg × = ppm 1 mol 1g 40.078 gCa 1000 mg × = 40.88 ppm 1 mol 1g Claimed wt% of Calcium in tablet: mass CaCO3 × 0.500 g CaCO3 × 1 mol CaCO3 1 mol Ca 40.1g Ca × × 100.1g CaCO3 1 mol CaCO3 1 mol Ca 1 mol CaCO3 1 mol Ca 40.078 of Ca × × = 0.2002g 100.09 CaCO3 1 mol CaCO3 1 mol Ca grams Ca2+ × 100% = wt% claimed total mass of tablet 0.2002 g Ca2+ × 100% = 11.6% 1.7258 g Mass of calcium and wt% of calcium: L EDTA × Molarity of EDTA × 1 mol Ca molar mass Ca 20 parts × × = mass Calcium 1 mol EDTA 1 mol Ca solution 0.0159 L × 0.0098 M × 1 mol Ca 40.078 Ca 20 parts × × = 0.1249g 1 mol EDTA 1 mol Ca solution 𝑤𝑡% = 𝑚𝑎𝑠𝑠 𝑐𝑎𝑙𝑐𝑖𝑢𝑚 × 100 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑡𝑎𝑏𝑙𝑒𝑡 . 1249𝑔 × 100 = 7.24% 1.7258𝑔 Conclusion: The purpose of this lab was to determine the calcium hardness of tap water as well as the content of calcium in a commercial antacid through several EDTA titrations. We determined the calcium hardness of the tap water to be about 38.50ppm. We determined the content of calcium in the antacid tablet to be about 7.6% which was somewhat lower than the manufacturer claim of 11.6%. We attributed this error to our difficulty determining the endpoint of our titrations.