Topic III – Periodic Table - Science - Miami
advertisement
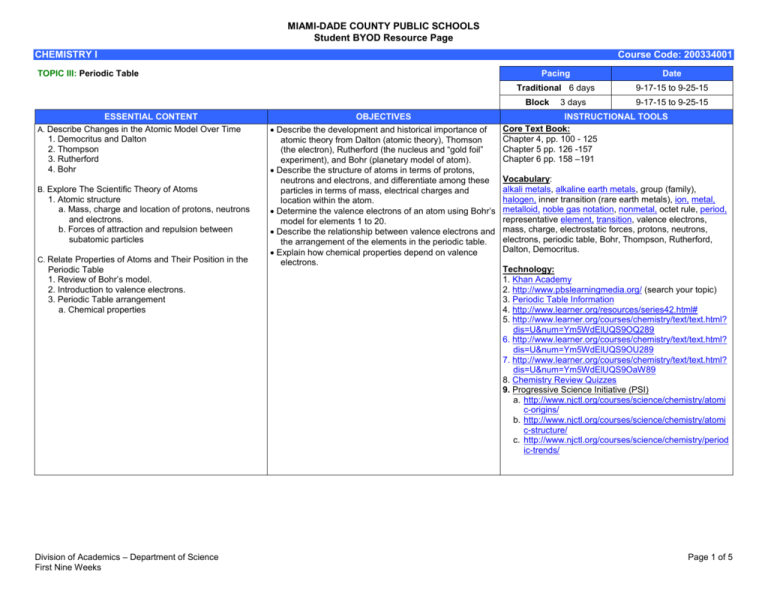
MIAMI-DADE COUNTY PUBLIC SCHOOLS Student BYOD Resource Page CHEMISTRY I Course Code: 200334001 TOPIC III: Periodic Table Pacing Date Traditional 6 days 9-17-15 to 9-25-15 Block ESSENTIAL CONTENT A. Describe Changes in the Atomic Model Over Time 1. Democritus and Dalton 2. Thompson 3. Rutherford 4. Bohr B. Explore The Scientific Theory of Atoms 1. Atomic structure a. Mass, charge and location of protons, neutrons and electrons. b. Forces of attraction and repulsion between subatomic particles C. Relate Properties of Atoms and Their Position in the Periodic Table 1. Review of Bohr’s model. 2. Introduction to valence electrons. 3. Periodic Table arrangement a. Chemical properties Division of Academics – Department of Science First Nine Weeks OBJECTIVES Describe the development and historical importance of atomic theory from Dalton (atomic theory), Thomson (the electron), Rutherford (the nucleus and “gold foil” experiment), and Bohr (planetary model of atom). Describe the structure of atoms in terms of protons, neutrons and electrons, and differentiate among these particles in terms of mass, electrical charges and location within the atom. Determine the valence electrons of an atom using Bohr’s model for elements 1 to 20. Describe the relationship between valence electrons and the arrangement of the elements in the periodic table. Explain how chemical properties depend on valence electrons. 3 days 9-17-15 to 9-25-15 INSTRUCTIONAL TOOLS Core Text Book: Chapter 4, pp. 100 - 125 Chapter 5 pp. 126 -157 Chapter 6 pp. 158 –191 Vocabulary: alkali metals, alkaline earth metals, group (family), halogen, inner transition (rare earth metals), ion, metal, metalloid, noble gas notation, nonmetal, octet rule, period, representative element, transition, valence electrons, mass, charge, electrostatic forces, protons, neutrons, electrons, periodic table, Bohr, Thompson, Rutherford, Dalton, Democritus. Technology: 1. Khan Academy 2. http://www.pbslearningmedia.org/ (search your topic) 3. Periodic Table Information 4. http://www.learner.org/resources/series42.html# 5. http://www.learner.org/courses/chemistry/text/text.html? dis=U&num=Ym5WdElUQS9OQ289 6. http://www.learner.org/courses/chemistry/text/text.html? dis=U&num=Ym5WdElUQS9OU289 7. http://www.learner.org/courses/chemistry/text/text.html? dis=U&num=Ym5WdElUQS9OaW89 8. Chemistry Review Quizzes 9. Progressive Science Initiative (PSI) a. http://www.njctl.org/courses/science/chemistry/atomi c-origins/ b. http://www.njctl.org/courses/science/chemistry/atomi c-structure/ c. http://www.njctl.org/courses/science/chemistry/period ic-trends/ Page 1 of 5 MIAMI-DADE COUNTY PUBLIC SCHOOLS Student BYOD Resource Page CHEMISTRY I SC.912.P.8.4 SC.912.P.8.5 Standard: SC.912.N.1.6 Course Code: 200334001 Element Builder Electron Configuration Video Standard: SC.912.N.1.7 Video Standard: SC.912.N.2.4 Video Standard: SC.912.N.2.5 Video Standard: SC.912.N.3.1 Video Exploring the Modern Periodic Table Using the Modern Periodic Table Chemical Properties of Metals Chemical Properties of Non-Metals Periodic Table of the Elements Louis de Broglie's Theory on Waves and Particles Niels Bohr's Model of the Atom The Evolution of the Periodic Table History of the Atom, Part 2 Division of Academics – Department of Science First Nine Weeks Page 2 of 5 MIAMI-DADE COUNTY PUBLIC SCHOOLS Student BYOD Resource Page CHEMISTRY I Course Code: 200334001 Early Atomic Discoveries Standard: SC.912.N.3.2 Video Standard: SC.912.P.8.3 Video Atomic Models by Thomson, Rutherford, Atomic Models by Bohr, de Broglie, and Schrodinger and Planck Video The Structure of Atoms Introduction to the Atom JJ Thomson Discovers Electrons Niels Bohr's Atomic Model Ernest Rutherford and the Structure of the Atom Electronegativity and Atom Size Atom Standard: SC.912.P.8.4 Image Article Video Standard: SC.912.P.8.5 Nucleus Electron Proton Neutron Overview, Metalloids Overview, The Non-metals Overview, The Halogens Overview, The Alkali Metals Overview, Alkaline Earth Metals Overview Transition Metals Overview The Other Metals The Interactive Periodic Table Overview, Noble Gases Overview Lanthanide Series Overview, Actinide Series Exploration Standard: SC.912.P.10.10 Video Division of Academics – Department of Science First Nine Weeks The Fundamental Forces Forces Working Together Page 3 of 5 MIAMI-DADE COUNTY PUBLIC SCHOOLS Student BYOD Resource Page CHEMISTRY I Course Code: 200334001 Video Smoot Point: Astrophysicist Links Latest Findings to Big Bang Theory Quest to Find Origins of the Universe: Huge Supercollider is Built in Switzerland The Chemistry of CO2: Carbon Dioxide From Metal to Plastic: Iowa State Chemist Works on Organic Semiconductors How Superconductivity and Superconductors Work New "Clock" Dates Materials Using Uranium Decay How Snowflakes Form (And Yes, Each One Is Different) Inside the Disaster Lab: Recreating Hurricanes, Fires The Blood Runs Cold? T-Rex's Didn't Scientists Closer to Reaching Nuclear Fusion How Atoms Bond: Ionic Bonds World's Largest Atom Smasher Fires Up in Chicago Computer Modeling Lets Drug Researchers See, Re-Design, Molecules Filtering Air Pollutants With Biowalls New Evidence of Water Once on Mars: Salt Crystal Traces Examined Smashing Success: Probe Hits Comet in Planned Collision Computer Modeling Lets Drug Researchers See, Re-Design, Molecules Cold Snap Threatens Crops Arctic Siberia Holds Crucial Key to Global Warming Balloons, Bubbles, and Dry Ice: Showing Students the Fun in Science You Won the Nobel Prize in Chemistry! Oops--Wrong Donald Cram Georgia Tech Chemist Designs Molecules That May Stop or Slow Effects of Alzheimer's "Green" NC State Chemist Looks for Cleaner, Safer Fuel Process Fabricating Fabric: Profile of Nylon How Atoms Bond: Ionic Bonds 21st-Century Gold Rush in Nevada South Africa is Vital Resource Source: Diamonds, Gold, Chromium, Platinum The Price of Gold in Mali What Howard Carter Saw: King Tut's Tomb Revisited Profile of Oliver Sacks, Chemistry Obsessive, Dreamer of Scandium Physics Prof Harold Goldberg is Professor of the Year The Chemistry of CO2: Carbon Dioxide Diamonds, Pencils and Buckyballs: A Look at Buckminsterfullerene The Dirt on Ammonia as a Cleaning Agent The Chemical Bond Between Cloves and Nutmeg The Science of Skis Molecule Profile: H2O - Water Producing Biofuels May Worsen, Not Lessen, Carbon Dioxide Emissions Black Carbon World's Largest Atom Smasher Fires Up in Chicago In 1977, Scientists Work Toward Building Fusion Furnace By Mid-90s New "Clock" Dates Materials Using Uranium Decay Scientists Closer to Reaching Nuclear Fusion Division of Academics – Department of Science First Nine Weeks Page 4 of 5 MIAMI-DADE COUNTY PUBLIC SCHOOLS Student BYOD Resource Page CHEMISTRY I Course Code: 200334001 Image LED There Be Light Periodic Table of the Elements (NIST, 2003) "The Evolution of the Periodic System" John Dalton: Chemical Atoms and Their Combinations (1808) Concentration of Dissolved-Solids in Aquifers, Streams of the Southwest How Did Earth's Atmosphere Form? Fairy Picture of Water - H2O The pH Scale Ammonia Molecules Straighten Up Atoms, Elements, Isotopes (NASA Genesis Mission Teacher Resource) Division of Academics – Department of Science First Nine Weeks Page 5 of 5








