ESSR_LBS_DrivesPlates_V02
advertisement
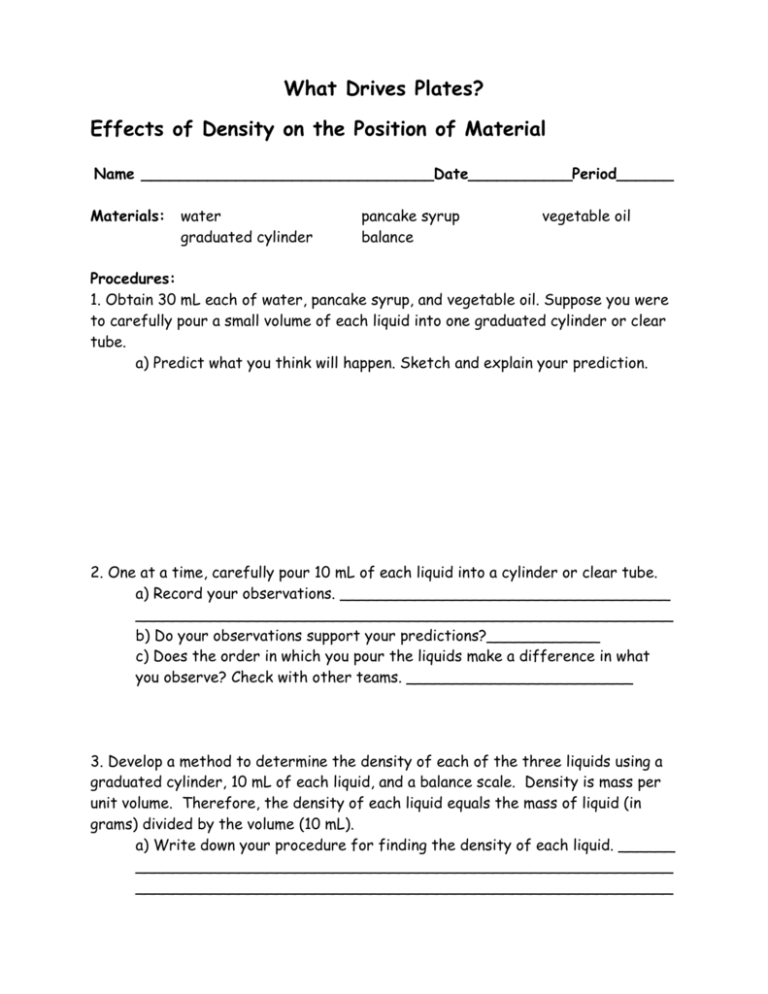
What Drives Plates?
Effects of Density on the Position of Material
Name _______________________________Date___________Period______
Materials:
water
graduated cylinder
pancake syrup
balance
vegetable oil
Procedures:
1. Obtain 30 mL each of water, pancake syrup, and vegetable oil. Suppose you were
to carefully pour a small volume of each liquid into one graduated cylinder or clear
tube.
a) Predict what you think will happen. Sketch and explain your prediction.
2. One at a time, carefully pour 10 mL of each liquid into a cylinder or clear tube.
a) Record your observations. ___________________________________
_________________________________________________________
b) Do your observations support your predictions?____________
c) Does the order in which you pour the liquids make a difference in what
you observe? Check with other teams. ________________________
3. Develop a method to determine the density of each of the three liquids using a
graduated cylinder, 10 mL of each liquid, and a balance scale. Density is mass per
unit volume. Therefore, the density of each liquid equals the mass of liquid (in
grams) divided by the volume (10 mL).
a) Write down your procedure for finding the density of each liquid. ______
_________________________________________________________
_________________________________________________________
b) Make a data table to record your measurements and calculations for each
liquid.
Substance
Mass
Volume
Density
c) After your teacher has approved your procedure, determine the density of each
liquid.
Teacher Approval: _______________________________
Conclusions:
4. Compare your calculations with your observations in Step 2.
a) Describe how the densities you calculated explain what you observed.
_________________________________________________________
_________________________________________________________
_________________________________________________________
b) If layers of materials of different densities within the Earth behave like
layers of liquids of different densities, what would you predict about the
position of the rock layers of different densities in the Earth? __________
_________________________________________________________
_________________________________________________________
Effects of Temperature on Density of a Material
Name _______________________________Date___________Period______
Materials:
bricks (2 per group)
beaker
corn syrup
balsa wood
candle
PRECAUTIONS: Follow your teacher’s safety advice about using a heat
source. Hot corn syrup can cause burns. Clean up spills immediately.
Procedures:
1. Place the bricks a few inches apart so the candle can slide between them.
2. Pour about a 5 cm thick layer of corn syrup into a Pyrex® beaker or wide
aluminum pan. Place the pan over the gap between the bricks. Light the candle and
slide it under the center of the pan.
3. Place three pieces of balsa wood on the syrup.
a) Predict what you think will happen to the wood as the corn syrup is
heated. Record your ideas in your notebook.
4. Observe the wood. Record any changes every 5 min for 20 to 30 min.
a) Observations: ____________________________________________
_________________________________________________________
_________________________________________________________
_________________________________________________________
b) Use diagrams to record the changes you observe. Sketches:
Conclusions:
1. Do your observations support your predictions? _____________________
_________________________________________________________
_________________________________________________________
2. What do you think caused the results you observed? _________________
_________________________________________________________
_________________________________________________________
Density of Earth Materials
Name _______________________________Date___________Period______
Materials:
Rocks: granite, basalt, sandstone, various others
graduated cylinder
balance
water
Procedures:
1. Collect samples of rock from your community and also obtain samples of granite,
basalt, and sandstone.
2. Predict qualitatively the density of the samples by answering the questions:
a) Which sample appears to be least dense? ________________________
b) Which appears to be most dense? _____________________________
3. Develop a method to find the density of each rock sample using the sample,
water, a graduated cylinder, and a balance scale. Density is mass per unit volume.
Therefore, the density of each rock equals the mass of rock (grams) divided by
the volume of rock (cubic centimeters). Remember: 1 mL = 1 cm3.
a) Write down your procedure for finding the density of each rock sample.
_________________________________________________________
b) Complete the data table to record your measurements and calculations.
c) After teacher approval, determine the density of each rock sample.
Teacher Approval: _______________________________
Sample
Mass
Volume
{Over}
Density
Conclusions:
4. Compare your calculations with your predictions and answer the following
questions.
a) How does the density of the rock from your community compare with the
density of granite?_________________________________________
b) How does the density of the rock from your community compare with the
density of sandstone?________________________________________
c) How does the density of the rock from your community compare with the
density of basalt?_________________________________________
G 87
G8
Forces Causing Subduction of Lithospheric Plates
Name _______________________________Date___________Period______
Materials:
rectangular tub
dish soap
flat plastic ruler
warm water
sponge
scissors
spoon
vinyl plastic
Procedures:
1. Partly fill a large, rectangular tub with warm water. Wait until any tiny air
bubbles have disappeared. The water has to be perfectly clear.
2. Very slowly and carefully, put a few ounces of liquid dish detergent in the water
and mix it slowly and carefully with a mixing spoon. If any soap bubbles or foam
remain on the water surface, scrape them off with a damp sponge.
3. Cut a piece of the vinyl plastic to be about six inches wide and about twelve
inches long. Trim a flat, clear-plastic ruler with the scissors to be the same
width as the plastic sheet. (The ruler should sink in water.) Tape the ruler to one
end of the plastic sheet.
4. Dip the ruler end of the plastic sheet into the water to a depth of about 1 cm.
Immediately place the plastic sheet on the water surface. Do this by holding the
ends up, and letting the sagging middle part of the sheet touch the water
surface first, to avoid trapping air bubbles under the sheet. Observe what
happens. Repeat this step as many times as you need to make careful
observations.
a) Record your observations. Include a description of the motion of the
plastic sheet in the water. _____________________________________
_________________________________________________________
_________________________________________________________
_________________________________________________________
Conclusions:
b) What is the force that makes the plastic behave as it did?___________
_________________________________________________________
c) How does this demonstration show what happens in a subduction zone?___
_________________________________________________________
_________________________________________________________