How do the densities of different liquids compare?
advertisement
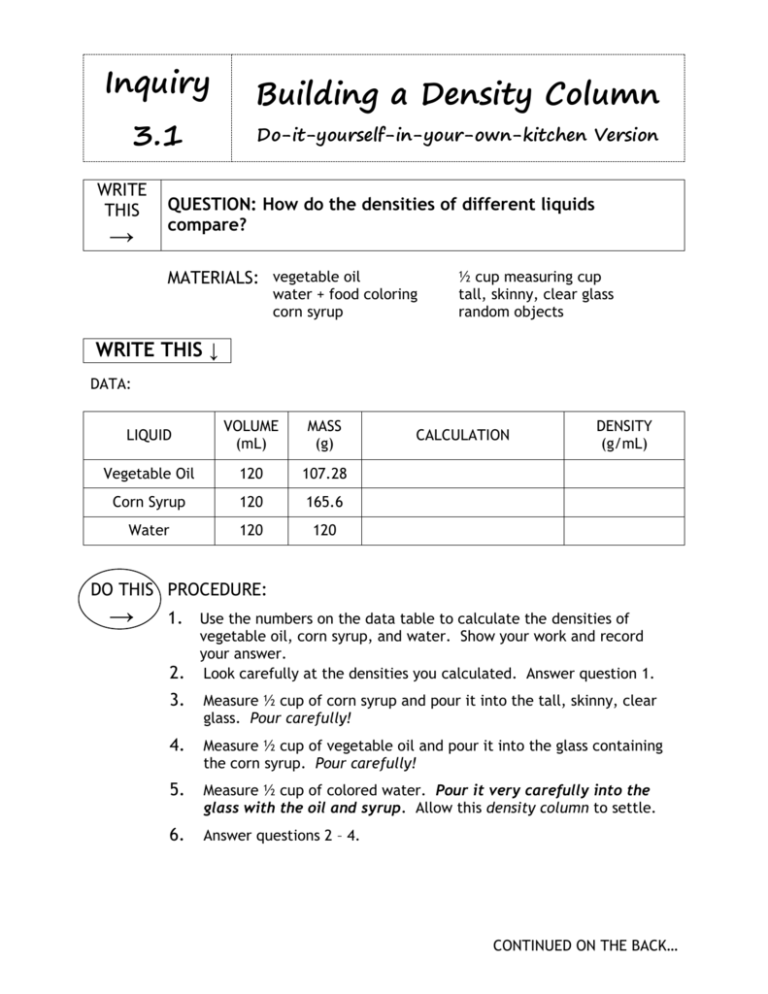
Inquiry 3.1 WRITE THIS → Building a Density Column Do-it-yourself-in-your-own-kitchen Version QUESTION: How do the densities of different liquids compare? MATERIALS: vegetable oil water + food coloring corn syrup ½ cup measuring cup tall, skinny, clear glass random objects WRITE THIS ↓ DATA: LIQUID VOLUME (mL) MASS (g) Vegetable Oil 120 107.28 Corn Syrup 120 165.6 Water 120 120 CALCULATION DENSITY (g/mL) DO THIS PROCEDURE: → 1. Use the numbers on the data table to calculate the densities of vegetable oil, corn syrup, and water. Show your work and record your answer. 2. Look carefully at the densities you calculated. Answer question 1. 3. Measure ½ cup of corn syrup and pour it into the tall, skinny, clear glass. Pour carefully! 4. Measure ½ cup of vegetable oil and pour it into the glass containing the corn syrup. Pour carefully! 5. Measure ½ cup of colored water. Pour it very carefully into the glass with the oil and syrup. Allow this density column to settle. 6. Answer questions 2 – 4. CONTINUED ON THE BACK… WRITE THIS → ANSWER THESE → Please use complete sentences 7. Choose several objects to drop in your density column. Suggestions: copper penny, eraser, rubberband, plastic pen cap, glass marble, cork, paperclip, etc. 8. Carefully drop your objects in. Draw and label each object in your drawing on question 2. QUESTIONS: 1. What do you predict will happen when you mix together the vegetable oil, corn syrup, and water? Explain your prediction and draw a picture of what you think these liquids will look like inside a graduated cylinder. 2. Draw a new picture of a graduated cylinder of what the liquids look like. Label each one and label their densities. 3. Do the liquids mix together (miscible) or form distinct layers (immiscible)? 4. What is the relationship between the density of the liquid and its position in the graduated cylinder?