Answer Key to week 11/10

NAME____ Answer Key __Date__________________
HOMEROOM_____________________
SCIENCE Answer Key week of Nov 10
Relax! Take a deep breath! Count backwards from 10! Now take your quiz!
1.
Which of the following describes what happens during a chemical reaction between two compounds? a) Some atoms are lost during the reaction b) Atoms in the reactants rearrange to form particles with different properties. c) A solid dissolves in a liquid d) New atoms form during the reaction.
2.
Reactants are listed on the left side of an equation. (Left, right)
3.
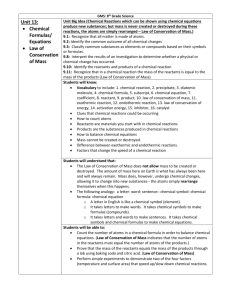
The Law of Conservation of Mass states that matter cannot be created or destroyed during a chemical reaction. (Matter, reactants)
4.
Compounds split apart to form smaller compounds in a decomposition reaction. (Combination, decomposition)
5.
Product is the substance made during a chemical reaction. (reactant, product)
6.
A sentence that describes a chemical reaction using symbols is called a chemical equation . (Chemical equation, chemical change)
True or False:
7.
False When a chemical change occurs, matter keeps the same chemical properties.
8.
True When matter changes state, it is a physical change.
9.
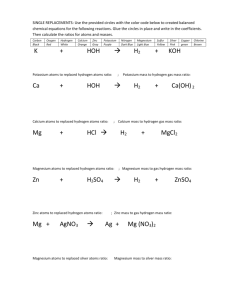
What kind of chemical reaction is represented by the chemical equation below? How can you tell?
AgNO
3
+ NaCl -> AgCl + Na NO
3
A replacement reaction (double replacement) is represented by this chemical equation. Atoms of the reactants have split and rearranged into new products.
10.
During a combination reaction, two hydrogen atoms combine with two oxygen atoms. How many hydrogen and oxygen atoms will be present in the product. Explain your answer.
There will be two atoms of hydrogen and two atoms of oxygen present in the product. The law of conservation states that no atoms are created or destroyed during a chemical reaction. Therefore in this chemical equation there will be the same amount of atoms on both sides of the equation.