3chemistryassignment
advertisement
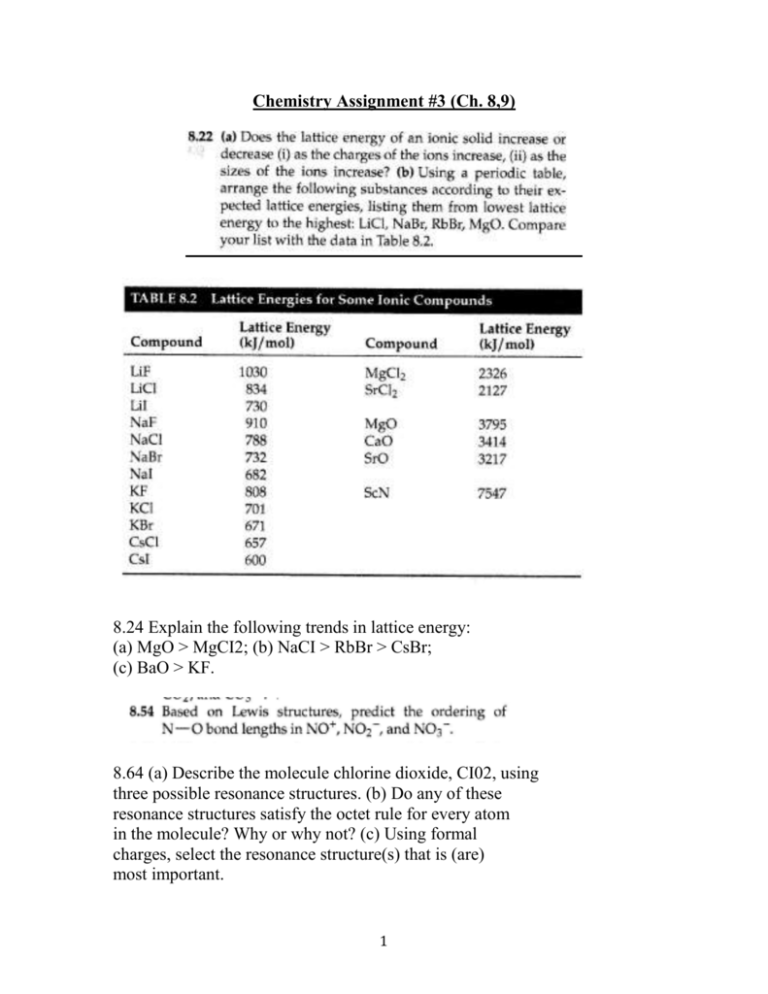
Chemistry Assignment #3 (Ch. 8,9) 8.24 Explain the following trends in lattice energy: (a) MgO > MgCI2; (b) NaCI > RbBr > CsBr; (c) BaO > KF. 8.64 (a) Describe the molecule chlorine dioxide, CI02, using three possible resonance structures. (b) Do any of these resonance structures satisfy the octet rule for every atom in the molecule? Why or why not? (c) Using formal charges, select the resonance structure(s) that is (are) most important. 1 8.68 Use bond enthalpies (Table 8.4) to estimate the enthalpy change for each of the following reactions: (a) 3 H2C=CH2(g) ~ C6H12(g) (the six carbon atoms from a six-membered ring with two hydrogen atoms on each carbon atom) (b) SiClH3(g) + 3 CI2(g) ~ SiCI4(g) + 3 HCI(g) (c) 8 H2S(g) ~ 8 H2(g) + S8(s) (See Figure 7.28. Strictly speaking, the average bond enthalpy values apply to species in the gas phase. The heat of formation of S8(g) is 102.3 kJ/mo!. Apply the needed correction in order to estimate the enthalpy change for the reaction as shown.) 8.99 The compound chloral hydrate, known in detective stories as knockout drops, is composed of 14.52% C, 1.83% H, 64.30% CI, and 19.35% 0 by mass and has a molar mass of 165.4 g/mol. (a) What is the empirical formula of this substance? (b) What is the molecular formula of this substance? (c) Draw the Lewis structure of the molecule, assuming that the CI atoms bond to a single C atom and that there is a C-C bond and two C-O bonds in the compound. 8.105 Consider benzene (C6H6) in the gas phase. (a) Write the reaction for breaking all the bonds in C6H6(g), and use data in Appendix C to determine the enthalpy change for this reaction. (b) Write a reaction that corresponds to breaking all the carbon-carbon bonds in C6H6(g). (c) By combining your answers to parts (a) and (b) and using the average bond enthalpy for C- H from Table 8.4, calculate the average bond enthalpy for the carbon-carbon bonds in C6H6(g). (d) Comment on your answer from part (c) as compared to the values for C-C single bonds and C=C double bonds in Table 8.4 (p.3). 2 9.26 Give approximate values for the indicated bond angles in the following molecules: 3 9.38 Dichlorobenzene, C6H4Cl2, exists in three forms (isomers), called ortho, meta, and para: JvCI ortho meta para Which of these would have a nonzero dipole moment? Explain. 9.42 (a) Draw a Lewis structure for silane (SiH4), and predict its molecular geometry. (b) Is it necessary to promote an electron before forming hybrid orbitals for the Si atom? (c) What type of hybridization exists in SiH4? (d) In one diagram, sketch two of the two-electron bonds formed between a hybrid orbital on Si and an H Is orbital. How would the other Si- H bonds be oriented relative to your sketch? 4 9.72 (a) The nitric oxide molecule, NO, readily loses one electron to form the NO+ ion. Why is this consistent with the electronic structure of NO? (b) Predict the order of the N -0 bond strengths in NO, NO+, and NO-, and describe the magnetic properties of each. (c) With what neutral homonucIear diatomic molecules are the NO+ and NO- ions isoelectronic (same number of electrons)? 9.88 Butadiene, C4H6, is a planar molecule that has the following carbon-carbon bond lengths: (a) Predict the bond angles around each of the carbon atoms, and sketch the molecule. (b) Compare the bond lengths to the average bond lengths listed in Table 8.5. Can you explain any differences? 5 6