PLANT PIGMENT ANALYSIS:
advertisement

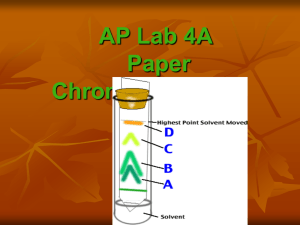
PAPER CHROMATOGRAPHY SEPARATES PLANT PIGMENTS Objectives: 1) Prepare an extract of plant tissue (e.g., leaves, flowers). 2) Apply the technique of paper chromatography as a method for separating individual plant pigments contained in plant tissue extracts containing pigment blends. 2) Describe the application of this technique to the study of plant pigments and develop related testable questions. 3) Generate ideas about ways to improve the technique to yield better results. INTRODUCTION TO PLANT PIGMENTS A "pigment" is simply a molecule that absorbs and reflects light. Recall that white light actually consists of many colors – you may have learned “ROY G BIV” in high school physics as a way to remember the colors of light that make up the white light of the “visible spectrum”. Different pigments appear different colors because they have differing abilities to absorb and reflect various colors of light. (A more thorough discussion of the light-absorbing properties of pigments will be presented in the Spectrophotometry lab.) The broad array of colors found in plant tissues such as leaves, flowers, and fruits, can be accounted for by the presence of literally thousands of different kinds of plant pigments. Through plant breeding and horticultural practices, humans have manipulated plants’ pigment producing capabilities to serve our own desires. News was made recently when a true blue rose cultivar was successfully created in Japan. In nature, color is an important attribute of plants that serves to attract pollinators to receptive flowers and signal fruit ripeness to seed dispersers. In some instances, colors may also serve to warn potential predators of poisonous or toxic substances contained in plant tissues. Color-producing pigments have other important roles in plants beyond regulating interactions with animals. Chlorophyll is a pigment that reflects green light, but absorbs red and blue wavelengths and is critical for the light reactions of photosynthesis. Flavonoids are an important class of plant pigments that block ultraviolet (UV) radiation that can damage cell proteins and DNA. Many flavonoids, including anthocyanins (a subcategory of flavonoids) have a role in the chemical defense of plants as they are toxic to many herbivores and pathogens – especially insects and fungi. As you may know from the popular media, there is currently a substantial research effort in place to explore the potential health benefits of plant pigments to humans. In popular literature, these plant-based compounds are often collectively referred to as “phytochemicals”; most are also pigments. Flavonoids, anthocyanins, and carotenoids are just some of the categories of plant pigments known to have antioxidant properties. “Antioxidant” is a general term used to describe any substance that has the ability to neutralize “free radicals” which cause cellular damage by removing electrons from surrounding molecules. Many lines of research suggest that consuming a diet rich in plant pigments may slow the process of cellular aging and reduce the risks of some types of disease, such as cancer, heart disease, and stroke. Cosmetic companies are even jumping on the “antioxidant bandwagon” by adding seductive blends of antioxidant-rich “botanical extracts” to their shampoos, makeups, and lotions in the hopes of prolonging our youthful glow! A few categories of pigments are listed below along with their characteristic range of colors. Pigment Type Anthocyanins (subclass of flavonoids) Anthoxanthins (subclass of flavonoids) Betacyanins Carotenoids Chlorophylls Xanthophylls (a subclass of carotenoids) Colors blue/purple/red yellow - ivory yellow - red/purple yellow - red greens ivory - yellow Some plant pigments you may be familiar with that are of current interest in nutritional and pharmaceutical research are listed below, though there are many more! Pigment anthocyanins Color blue/purple/red beta-carotene orange/yellow curcumin yellow Found in berries, grapes, red peppers, beets, eggplant, plums carrots, pumpkin, sweet potatoes, citrus, papaya, melon, squash turmeric lutein lycopene yellow/orange red kale, broccoli, spinach tomatoes, watermelon, red grapefruits zeaxanthin yellow corn PAPER CHROMATOGRAPHY METHOD Step 1: Prepare chromatography papers. Cut the chromatography paper into strips following dimensions suggested by your instructor. Draw a fine pencil line across (but not all the way across) the strip about 2-3 cm from one end - this is the "origin". Handle the papers by the edges, taking care to touch them as little as possible - oils from fingertips can interfere with the migration of pigments up the paper. the “solvent front” is the position of the liquid solvent on the chromatography paper at any given time. the solvent will gradually move from the bottom toward the top of the paper, carrying dissolved pigments with it. stop the chromatogram before the solvent front reaches the top of the paper and mark the location. you will use this distance to calculate Rf mark the location of each pigment at the time the chromatogram is stopped origin (where you apply the pigment extract) Step 2: Prepare your plant extract. In this procedure, we are interested in the “qualitative” assessment of the presence of individual pigments, we are not quantifying them (determining how much of a pigment is present). For this reason, exact measurements of plant tissue and extracting solvent are not necessary. (In the Spectrophotometry lab, we will be quantifying plant pigments, so careful measurements will be necessary.). Place the plant tissue of interest into a mortar (a few leaves or flowers). Add a small amount of ethanol to the plant tissue and grind completely with a pestle to release the pigments into solution. Continue adding ethanol (or plant tissue) as necessary to create a few milliliters of very dark extracted liquid. Your goal is to create a highly concentrated solution but avoid a paste-like consistency. Step 3: Load the extract onto the chromatogram. Dip a capillary tube into the liquid portion of your extract. (Your extract may contain fragments of plant tissue which will clog the capillary tube. To minimize this, tilt your mortar slightly to allow the liquid fraction to run away from the solids.) Allow the extract to migrate up the capillary tube. Dab the end of the capillary onto the origin of your chromatography paper. The extract will move out of the capillary tube onto the paper as it is absorbed into the paper fibers. Allow the extract to dry completely on the paper, then repeat with another load of extract. In order to concentrate the pigments on the paper, you will need to apply several loads of extract (probably 4 - 6). After each loading, wait until the paper is fully dry before applying the next load. Depending on the type of sample you have and the amount of water in it, it may take several minutes for the sample to dry (2 - 10 minutes). You may use a hairdryer on the lowest/coolest setting to speed the process. Do not proceed to step 3 until your sample is completely dry. Don’t forget: you will need to prepare 1 chromatogram for each chromatography solvent you will be testing. Your instructor will let you know which chromatography solvents should be tested for a given plant tissue. Use the same extract to prepare all the chromatograms. Step 3: Set the chromatogram in the chromatography solvent. Place your paper strip into the solvent container provided with the origin end down. Make sure that the level of the solvent is below the origin on your chromatogram – you do not want to submerge the origin in the solvent. Check the chromatogram frequently to observe the movement of solvent and pigment up the chromatography paper. WARNING: The petroleum ether/acetone solvent is highly flammable and can be dangerous if inhaled. Take care to avoid inhaling the fumes as much as possible and keep clear of any flame, spark, or other ignition source!! Step 4: Stop the chromatogram and record your results. When the solvent "front" is within 2-3 cm from the top of the paper, remove the chromatogram. Use a pencil to quickly mark the location of the solvent front. Allow the chromatogram to air dry, then trace and label the pigments you observe (they will fade over time). Step 5: Identify your pigments. Calculate the Rf value (described below) for each pigment in each chromatography solvent tested. Consult your instructor (or reference provided) about the identity of the pigments you isolated – record all your data in your notebook. IDENTIFYING PIGMENTS ISOLATED BY PAPER CHROMATOGRAPHY Different pigments have different sizes, shapes, and physical properties (e.g., different solubilities in our chosen solvent). As a result, different pigments will move at different rates up the chromatography paper allowing them to visibly separate from one another. Once the pigments are separated, they can be identified by a variety of methods. One way to determine the identity of a pigment is to physically remove it from the paper and assay it by another method. For example, we could elute (remove) a pigment from the chromatography paper by dissolving it in another solvent, such as ethanol and measuring its absorption spectrum using the spectrophotometer. The resultant spectrum could be compared to the known spectra for different pigments by searching in an appropriate reference manual. An alternative method is to calculate the “Rf value”, a ratio representing the distance a pigment travels relative to the distance the chromatography solvent travels. Again, we can then match the Rf value and color of our unknown pigment to known values recorded in a reference manual. Rf value = distance from origin to pigment distance from origin to solvent front Remember – because pigments vary in their solubility in different solvents, the Rf value for a given pigment is tied to the chromatography solvent. In other words, chlorophyll b will have an Rf value in petroleum ether/acetone that is different from its Rf value in BAW. POSTLAB QUESTIONS: 1. Sketch each resulting chromatogram into your lab notebook (or scan it/take digital photos and paste it in) and construct an associated table that summarizes your data. Give each table a title that identifies the plant and chromatography solvent used. Include in your table: the number of pigments isolated with each chromatography solvent (e.g., “pigment 1”, “pigment 2”, etc. . .), a color description of each pigment, its Rf value, and its likely identity. 2. What was the initial color of the plant extract(s) you used? What is the relationship between the colors of the pigments you isolated and the color of the original extract? 3. Why is it important to stop the chromatogram before the solvent front reaches the top of your chromatography paper? What would happen to your chromatogram if you let it run too long? 4. In some cases, two or more pigments may overlap each other on the chromatogram, making it difficult to isolate and identify them. What are some things you could do to modify the method to improve pigment separation (distance between pigments)?