Full Text
advertisement

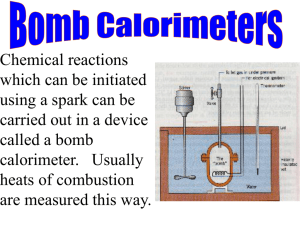
Final project: Heat of combustion of cysteine BE210 Group W4 Kate Guterl Soe Ahn Laura Swibel William Altman Abstract A high sulfur containing amino acid, cysteine was used to determine the accuracy of the non-adiabatic Parr Bomb Calorimeter in measuring the heats of combustion of such compounds. The result shows that sulfur-containing compounds could be accurately measured using the bomb calorimeter. Our measurements had a .02% deviation, showing the precision of our technique. The heat of combustion was found to be 449 kcal/mole. This value was calculated taking into account all corrections that needed to be made for the burning of the fuse wire and the formation of nitric and sulfuric acid. When calculating the heat of formation without making these corrections, the heat of combustion was within 0.6% of the reference value. We assume that the reference value was calculated without any corrections. To verify that our measurements were accurate, we tested a sucrose and sulfur mixture with a similar sulfur composition to cysteine. The value was within 0.53% of the theoretical value. When pure sucrose was tested it was within 0.7% of the given literature value. This verified the accuracy of the Parr bomb calorimeter in measuring the heat of combustion of sulfur containing compounds. 2 Background Cysteine is a high sulfur containing amino acid synthesized by the liver. (See fugure 1.) It is an important precursor to glutathione, one of the body’s most effective antioxidants. Glutathione protects the liver and brain from the damaging effects of cigarettes and alcohol, protects red blood cells from oxidative damage, and aids in amino acid transport. Cysteine has been shown to be effective in prevention and the treatment of atherosclerosis, heart attacks, cancer, osteoarthritis, and rheumatoid arthritis. It enhances the immune system and tissue healing after surgery or burns. Cysteine also assists in the supply of insulin to the pancreas, which is needed for the assimilation of sugars and starches. Cystine is a more stable form of cysteine that contains two cysteine molecules. (See figure 2.) The body is capable of converting one to the other as required. In metabolic terms they can be thought of as the same. Cystine is found abundantly in hair keratin, insulin, and certain digestive enzymes. By reducing the body's absorption of copper, cystine protects against copper toxicity, which has been linked to behavioral problems. It is also used to break down mucus deposits in illnesses such as bronchitis and cystic fibrosis. (http://www.smartbasic.com/glos.aminos.dir.html) Figure 1: Structure of a cysteine molecule Figure 2: Structure of a cystine molecule Biological organisms convert chemical energy to energy they can utilize through biochemical chain reactions that are thermodynamically equivalent to combustion. The heat of combustion of an energy rich metabolite represents the maximum energy that can 3 be produced through any bioenergetic process. It is a crucial part in understanding the efficiency of such a process. The standard heat of combustion of a compound is defined as the energy released by burning it completely in oxygen at 298K and 1atm. It is assumed that any compounds containing C, H, and N are converted to CO2 (g), H2O (l), and N2 (g). One way to measure the heat of combustion of a solid compound is by bomb calorimetry. A weighed mass of the compound is burned to completion inside a "bomb," which has been pressurized with oxygen and immersed in water in the calorimeter allowing the temperature rise of the water following the combustion to be measured. In the simplest model possible, in which all the energy released by the reaction goes into heating the water, the heat of combustion can be obtained from the energy balance: Qreaction = masssample * Hcomb,sample = masswater * Cp,water * twater Equation 1 This idealized relationship cannot be applied to real experimental values since some of the energy goes into other processes such as: Heating the metal of the bomb itself; Creating non-standard reaction products, such as nitric acid rather than N2 gas; Burning something other than the sample, such as the fuse wire used to start the reaction; Energy is lost through the walls of the calorimeter if it is non-adiabatic. These processes will cause the final temperature of the water to change. Thus, to obtain a more accurate result of the heat of combustion, all the energy added to the system and all the energy lost from the system has to be calculated. This can be done by calibrating the calorimeter. For the bomb calorimeter, the calibration is done by performing an experiment using a standard sample, such as very pure benzoic acid, whose heat of combustion is known very precisely. The energy of the calorimeter, W, can be found by the following equation. 4 W Hmet 1e 2 Equation 2 H: heat of combustion of benzoic acid m: mass of the benzoic acid sample e1: correction for heat of formation of nitric acid = ml of 0.0709N alkali solution used in the acid titraton. e2: correction for heat of combustion of the firing wire = (2.3)*cm of fuse wire burned t: net corrected temperature rise (see equation 4) Given, W, the heat of combustion of an unknown sample can be found by using following equation: masssample* Hcomb = W*twater Equation 3 In addition to the calibration constant, there are several other factors that need to be considered for corrections: 1. During the combustion process the high temperature and pressure conditions existing within the bomb are sufficient to drive several possible secondary chemical reactions, in addition to the expected combustion. For samples containing sulfur or nitrogen, the reaction usually leads to the exothermic formation of sulfuric or nitric acid, respectively. The heating of the calorimetry system by the combusted sample is, therefore, supplemented by the energy released by such exothermic reactions. To correct our energy balance for this effect the energy due to the reactions must be subtracted from the right-hand side of Equation 3. Note that one source of nitrogen is always present in this experiment: the nitrogen in the residual air of the bomb. 2. The heat generated by the combustion of the fuse wire varies from trial to trial; therefore, a correction term is required for the portion of the fuse wire burned. The correction for the 5 heat generated by the combustion of the fuse wire should be subtracted from the right-hand side of Equation 3. 3. An additional correction is required to account for the transfer of heat between the calorimeter and the surrounding environment. During the temperature rise period some of the heat stored in the calorimeter is transferred to or lost to the surroundings (when the temperature of the calorimeter is above room temperature). Because of the exchange of heat with the surroundings, this type of calorimeter is called a non-adiabatic calorimeter. In contrast to this relatively simple calorimetry system, adiabatic calorimeters, which are more elaborate and expensive, use a control system to match the temperature of an outer "heated" jacket to the temperature of the water so there is no heat exchange between the water and the surroundings. The temperature rise correction was calculated using Equation 4, in which, a : time of firing b : time when the temperature reaches 60% of the total rise c : time at the beginning of the period in which the rate of the temperature became constant ta: temperature at a tc: temperature at c r1: rate at which temperature was rising during the 5-minute period before firing r2: rate at which the temperature was rising during the five minute period after time c. t = tc – ta-r1(b-a) – r2(c-b) Equation 4 Thus the gross heat of combustion becomes: 1e 2e3 Hc t*W em Equation 5 e1: correction for heat of formation of nitric acid = 10* (ml of 0.709N alkali solution used in the acid titration) e2: correction for heat of formation of sulfuric acid = 13.7* mass of sample * percentage of sulfur in the sample 6 e3: correction for burning of the fuse wire W: energy equivalent of the calorimeter determined under standardization m: mass of sample t: corrected temperature rise Since corrections must be made for the formation of nitric and sulfuric acid, cysteine and cystine compounds were chosen specifically to test the method of determining the heat of combustion of sulfur containing compounds with a bomb calorimeter. 7 Material and Apparatus 1. Parr Instrument Model 1341 Oxygen Bomb Calorimeter, including stirrer, precision thermometer, and associated components. 2. Parr Instrument Model 1108 Oxygen Combustion Bomb 3. High Pressure oxygen cylinder, equipped with Model 1825 filling connection for bomb 4. Parr Model 2901 Ignition unit 5. Parr 45C10 nickel chromium fuse wire 6. Parr Pellet press 7. Associated components for test: sample cups, ignition wire, stands for bomb head and calorimeter cover, thermometer magnifier. 8. 0.0709 N, 0.709N Sodium Carbonate Solution 9. Methyl Red Indicator 10. 1000 micro-L Micro Pipette 11. Top-loading 5kg balance with a resolution of 0.1g for measuring the mass of water 12. Balance with a resolution of 1mg for measuring the mass of the cysteine sample. 13. Desiccator with drierite 14. Burette, 50ml 15. Ruler 16. Benzoic Acid for standardization 17. Deionized Water 18. Cysteine 19. Cystine 8 Procedure 1) Standardization: The oval bucket of the calorimeter was filled with 2000g of deionized water measured with a balance. The water temperature was kept approximately 1.5oC below the room temperature. About 1.0 gram of benzoic acid powder was measured and made into a pellet with the Parr pellet press. Then the pellet was placed in a sample cup. 10 cm of the fuse wire was cut and attached to the two electrodes such that about 2 mm of the wire touches the pellet. 1 mL of distilled water was put inside the bomb as a sequestering agent. The bomb was closed and filled with oxygen until the pressure within the bomb reached 25atm. Then the bomb was placed in the oval bucket of the calorimeter. The ignition wires were connected to the bomb, and the thermometer and the stirrer shaft were submerged in the water through the calorimeter cover. The stirrer shaft and the motor were connected by a stirrer drive belt. The motor was turned on and the temperature of the water was measured every minute for 5 minutes. After 5 minutes, the bomb was ignited using the Parr ignition unit. Then the temperature was measured every 30 to 60 seconds until it reaches a constant temperature for 5 minutes. After the last temperature reading, the bomb was removed from the calorimeter, and the knurled knob on the bomb head was opened to release the gas pressure before removing the cap. The amount of wire burned was measured by subtracting the amount left from the original 10 cm. The amount of nitric acid formed was determined by titrating the acid left in the sample cup with 0.0709 N sodium carbonate solution which was mixed with methyl red indicator. The energy equivalent of the calorimeter (W) was calculated using equation 3. Five independent trials were carried out for the standardization. 2) Measuring the heat of combustion of cysteine: The procedure was the same as in part 1 except that a 0.750g pellet of cysteine was used in place of benzoic acid and a 0.709N sodium carbonate solution, ten times more concentrated than the first titration solution, was used due to the large amount of acid formed. The trial was repeated two more times, and the results were plotted. The gross heat of combustion can be found using Equation 5. 9 The average value from standardization was used for W in calculation. The procedure follows the Parr “Operating Instructions for the 1341 Oxygen Bomb Calorimeter.” 3) Measuring the heat of combustion of cystine: The procedure was the same as the procedure for the combustion of cystiene. 4) Measuring the heat of combustion of sucrose and sulfur mixture: A sucrose and pure sulfur mixture with approximately 26 wt % sulfur was made into a pellet and combusted following the procedure described above. The weight percentage was determined based on the fact that cysteine has about 26.4 wt % sulfur. 10 Results Graph 1, below, shows the increase in temperature of the water in the bomb calorimeter as the cysteine was burned. All trials had very similar looking graphs with respect to the temperature response to combustion. The temperature rose slightly in the five minutes before combustion. Immediately after the sample was fired the temperature increased very rapidly. The graphs for the combustion of benzoic acid and cystine looked similar as well. Knowing the heat of combustion of benzoic acid, according to the Parr Operating Manual, the energy equivalent of the calorimeter was determined. As shown in Table 1, the energy of the calorimeter, W, was found to be 2448.95 15.21 cal/oC, a 0.6% standard deviation. This value was used in the calculation of the heat of combustion of all samples in the bomb. Trial 1 2 3 4 5 Average Stand dev % dev Wcalor. (cal/oC) 2443.36 2435.45 2462.41 2434.58 2466.97 2448.95 15.2077 0.6211 Graph 1: Plot of water temperature versus time Cysteine 25.6 25.4 Temperature (C) Table 1: Energy Equivalent of the Calorimeter 25.2 25 24.8 24.6 24.4 24.2 24 0 5 10 Time (min) 15 20 First, we found the heat of combustion of cysteine. Correcting for the formation of nitric and sulfuric acid, and the burning of the fuse wire, we determined the heat of combustion of cysteine to be -448.6 0.114 kcal per mole of cysteine. There was a 0.03% standard deviation. The results are shown in Table 2. 11 When the corrections for nitric and sulfuric acid and the burnt fuse wire were not taken into account, the heat of combustion of cysteine was calculated to be -536.8 2.175 kcal/mol, a 0.4% standard deviation. The results are shown in Table 3. Table 2: Heat of Combustion of Cysteine -H (kcal/mole) 1 448.5 2 448.7 3 448.4 Average 448.6 Stand dev .114 % dev 0.0254 Confidence limit -448.8 to –448.3 kcal/mol t Stat -0.00051 t Critical 4.303 Trial Table 3: Heat of Combustion without corrections e1,2 and 3 Sabbah and Minadakis, 1981 -H = 537.5 kcal/mol Trial -H (kcal/mole) 1 536.0 2 535.2 3 539.3 Average 536.8 Stand dev 2.18 % dev 0.405 Confidence limit -542.2 to –531.4 kcal/mol t Stat -0.5386 t Critical 4.303 With all correction factors included, the heat of combustion of L-cystine is -885.4 11.34 kcal per mole of L-cystine. The percent deviation is 1.28%, as shown in Table 4. Without the corrections made for sulfuric and nitric acid, and the burnt fuse wire, the heat of combustion of L-cystine is -1027.1 10.59 kcal/mol. The percent deviation is 1.03. The results are shown in Table 5. As shown in Table 1 through Table 5, the results from each trial fell within the 95% confidence limits for their respective trials and t Stat values were always less than t Critical, meaning the values obtained from each trial were not statistically significantly different from one another. 12 Table 4: Heat of Table 5: Heat of Combustion Combustion of L-Cystine without corrections e1,2, and 3 Trial H (cal/mole) 1 881347.4 2 898242.4 3 876681.2 Average 885423.7 Stand dev 11343.87 %dev 1.281 Confidence limit -857244 to -913603 cal/mol t Stat -5.09E-09 t Critical 4.304 Sunner, 1946 H = 1015300 cal/mol Trial H (cal/mole) 1 1023332 2 1039081 3 1018929 Average 1027114 Stand dev 10594.97 %dev 1.031 Confidence limit -1000795 to –1053433 T Stat 1.931 t Critical 4.303 For the last trial of this experiment, sucrose and sulfur mixture was combusted. The heat of combustion of this mixture with 27.7 wt % sulfur was found to be -3469.7 cal/g as shown in table 6. Only one trial was performed due to constriction in time. Table 6: Heat of combustion of sucrose & sulfur mixture mass sucrose + sulfur (g) Mass of sucrose (g) mass of surfur (g) W cal/C e1, nitric mL e2, sulfuric e3, fuse wire t (corrected) -Hcomb (cal/g) = 0.74 0.535 0.205 2443.95 150.5 281.535 14.26 1.233 3469.7 13 Discussion The average value found for W (energy equivalent or the calorimeter) was 2448.95 cal/oC 0.6 % deviation. The small percent deviation represents the high precision in this result. The suggested value for W given in the BE 209 Lab manual was 2426 cal/oC. Although our value is within 1% of the suggested, the given W does not lie within the 95% confidence range of our value (2430.1kcal/oC < W < 2467.8 kcal/oC). The small standard deviation value led to a very narrow 95% certainty range. Thus, the suggested W was outside of the range. The bomb calorimeter is an accurate way to measure the heat of combustion of sucrose, as we discovered previously in the BE lab (Lab02.doc). Additionally, due to the high pressure of oxygen in the bomb, the products of a normal combustion reaction are not always the final products of the reaction. Upon combustion cysteine and cystine, the products of combustion, NO2 and SO2, oxidized to nitric acid and sulfuric acid. In the bomb, the temperature of the water rose due to these oxidations. To find the heat of combustion of sulfur and nitrogen containing samples, the correction must be made for the formation of sulfuric and nitric acid. This experiment proved that the corrections can be made accurately and that the bomb calorimeter is, in fact, useful for measuring the heat of combustion of compounds that contain sulfur and nitrogen, in addition to those that do not. Two sources available to us with the heat of formation of cysteine and cystine were http://webbook.nist.gov and The Handbook of Biochemistry (edited by Herbert A. Sober, Ph.D.) The values from the two sources did not have values similar to each other or to ours. The source from the web, http://webbook.nist.gov, quoted a value of -537 kcal per mole of cysteine from “Sabbah and Minadakis, 1981.” The average value calculated from this experiment was -449 kcal per mole of cysteine. Sabbah and Minadakis also measured the heat of combustion of cysteine by bomb calorimetry. Upon further investigation of the results of this experiment, it was discovered that if no corrections were made for the formation of nitric acid or sulfuric acid or the burning of the fuse wire, our average value for the combustion would be -537 kcal per mole of cysteine. Without the corrections made, our value (-536.8 2.18 kcal/mol) was not statistically significantly different than the value measured by Sabbah and Minadakis (see table 2). It is hypothesized that 14 Sabbah and Minadakis did not account for the corrections that were accounted for in this experiment. (The information was not available to us.) Also, the source from the web, http://webbook.nist.gov, quoted a value of -1015 kcal per mole of cystine from “Sunner, 1946.” The average value calculated from this experiment was -885 kcal per mole of cystine. Sunner also measured the heat of combustion of cystine by bomb calorimetry. As with cysteine, if no corrections were made for the formation of nitric acid or sulfuric acid or the burning of the fuse wire, our average value for the combustion would be -1027 kcal per mole of cystine. Without the corrections made, our value (-1027 10.6 kcal/mol) was not statistically significantly different than the value measured by Sunner (see table 5). The value we used from the web site was the recalculated value corrected by Cox and Pilcher using Sunners data. In “Thermochemistry of Organic and Organometallic Compounds,” Cox and Pilcher mentioned that corrections for the formation of sulfuric acid in the dilute state and the combustion in the non-standard state were made; however, exaclty how they made these correction is referenced to a source that is currently unavailable. They did not make corrections for the formation of nitric acid. In our experiment, ignoring the formation of nitric acid increases the heat of combustion value by about 9%, therefore the correction factor is crucial in finding the accurate heat of combustion. (Cox and Pilcher recalculated the heat of combustion of cysteine, and found the same value as Sabbah and Minadakis, so we assume that the heats of combustion were calculated in the same way, but somehow using different correction methods than ours.) Another source, the Handbook of Biochemistry, was consulted due to the difference in the heats of combustion found by Sabbah and Minadakis and Sunner and in this experiment. The Handbook of Biochemistry lists –394.6 kcal/mol as the heat of combustion of cysteine and –724.6 kcal/mol as that of cystine. These values differ significantly from our values found for the heat of combustion of cysteine and cystine as well as from those found by Sabbah and Minadakis and Sunner. According to the Handbook of Biochemistry edited by Herbert A. Sober, Ph.D. (National Institutes of Health) the values of the heats combustion for cysteine and cystine may not be accurate. Sober writes, “no reasonable choice could be made between apparently equally reliable heats of combustion,” indicating possible errors in the values obtained from this source. 15 Sober continues with more information relating to the reliability of the values he lists in the Handbook. He writes, “This table is intended to be comprehensive rather than selective. All heats of combustion that have come to the compiler’s attention have been reported for a compound.” It should also be noted that, “Most of the heats of combustion were originally reported as the enthalpy change for the combustion reaction with all gases at Pressure = 1 atm. No correction has been made for further expansion of the gases” (Sober). This may account for the difference in the heat of combustion values found. Results from our previous lab show the average value for heat of combustion of sucrose to be -3933.49 20.70 cal/g. This was within 0.7% of the known literature value of -3941.5 cal/g. The accuracy of the experimental value was 99.5 0.2%. The average percent error in three trials was 0.42%, and the standard deviation was 0.5% of the average value. Systematic error lead to a +/-15.8 calorie error in the thermal value. All of these statistics show the high accuracy and precision in the measurement of sucrose with the Parr Bomb Calorimeter. The heat of combustion of the sucrose and sulfur (27.7 wt %) mixture was tested to observe the effect of sulfur in the sample. Knowing weight percent composition of the mixture and the heats of combustion for sucrose and sulfur, (-3941.5cal/g and -2211.65 cal/g, respectively) the expected value for the heat of combustion was found to be -3462 cal/g. The value obtained from the experiment was -3469.7 cal/g. The experimental value is within 0.53% of the expected value. The presence of sulfur did not affect the accuracy of the results, indicating that the procedure and calculations to measure sulfur containing compounds is justifiable. The high accuracy of the trial of the sulfur/sucrose pellet combined with the high precision obtained through cysteine/cystine experiments show that the Parr bomb calorimeter can be used as a reliable method to measure the heat of combustion of sulfur containing compound. The sources of systemic errors in this experiment were the measurement of the fuse wire, the mass of benzoic acid, cysteine, and cystine, the titration of the acids formed, and temperature reading. The error in the measurement of the fuse wire was estimated to be +/-0.5 millimeters leading to a +/-0.115 calorie error in the thermal value obtained. The error in the measurement of the benzioc acid, cysteine, and cystine pellet 16 was estimated to be +/-0.0001g leading to a +/-0.34 calorie error. The titration of acid involved error in the collection of the acid from the bomb for titration as well as error of +/-0.5 mL in the measurement of the titration that leads to an error of +/-0.5 calories. Temperature readings consisted of an error of +/-0.005oC that leads to a +/-12 calorie error. The energy equivalent of the calorimeter (W) contributed to an additional error of 15.2 calories. Error must also be considered in the measurement of the 2000-mL of distilled water used in the calorimeter. This error is estimated to be +/-1 mL that leads to a +/-2.8 calorie change in the thermal value obtained. The temperature of the 2000 mL of water inside the jacket of the calorimeter and the ambient room temperature were approximately within 1.50C before firing which limits the error in the thermal value obtained according the Parr Operating Instructions. The total systematic error based on the above error estimates is a +/-31 calorie change in the thermal value obtained. One random error source was the presence of impure compounds within the highpressure cylinder such as dust or oil from fingers. Also, although the formation of nitric acid and sulfuric acid compounds are taken into account, there is the possibility of formation of other compounds as air is not solely oxygen and nitrogen. In addition, there were traces of residue at the bottom of the bomb after the combustion took place. 17 Reference 1) Smart basic : http://www.smartbasic.com/glos.aminos.dir.html 2) NIST (National Institue of Standard Technology) chemistry webbook: http://webbook.nist.gov 3) Cox, J.D.; Pilcher, G, Thermochemistry of Organic and Organometallic Compounds, Academic Press, New York, 1970, 1-636. 4) Herbert A. Sober, Handbook of Biochemistry 18 Appendix Table A-1. Sample calculation of the heat of combustion of cysteine. Cysteine Trial 1 mass cysteine 0.75 W cal/g 2443.95 e1, nitric mL 250.5 e2, sulfuric 271.26 e3, fuse wire 19.55 Hcombcysteine= 448.5 kcal/mol 19