Supplementary Figure Legends - Word file (27 KB )
advertisement
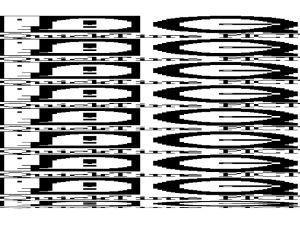
Legends of supplementary figures Figure S1. The elution profiles of PAZ domain alone and PAZ-9-mer RNA complex on superdex 75. Blue and red curves strand for the absorbance at 280 and 260 nm, respectively. The protein alone has a higher absorbance at 280 nm than that at 260 nm, whereas the protein-RNA complex shows reverse behavior. Compared to the protein standards, the elution peak of PAZ alone corresponds to that of an 18 kD species, suggestive of a monomeric protein. By contrast, the elution peak of the PAZ-9-mer RNA complex corresponds to a 49 kD species, suggestive of 2:2 complex formation. Figure S2. Stereo view of structural superposition of PAZ domains. Red-colored backbone represents our crystal structure of the PAZ domain from human eIF2cI bound by siRNA-like duplex. Green-colored backbone represents the structure of free PAZ domain of Drosophila Argonaute I solved by NMR (Yan et al, 2003), which aligns against our structure with1.44 Å root-mean-square deviation (rmsd) for 109 aligned Ca atoms. Blue-colored backbone represents the structure of free PAZ domain of Drosophila Argonaute II solved by NMR (Lingel et al, 2003), which aligns against our structure with 1.79 Å rmsd for 87 aligned Ca atoms. Superposition was made in PyMOL using a combination of sequence and structural alignment. The C-terminal end of our PAZ construct is extended from the core scaffold and does not form any secondary structure element. The interaction of the C-terminus of our PAZ construct with RNA may nevertheless have functional implications in the context of the full-length protein, given that it could form a short -sheet with a longer N-terminal segment, as observed in the PAZ domain of Drosophila Argonaute I (Yan et al, 2003). Figure S3. The sequence alignment of PAZ domains. The aligned sequences (genebank id) are in the order of Human eIF2C1 (6912352), Human eIF2C2 (29171734), Drosophila Argonaute 1 (17647145), Drosophila Argonaute 2 (24664668), Human HIWI (33563234), Drosophila PIWI (17136736), Tetrahymena TWI1(21671127), Human Dicer (29294651), Drosophila Dicer1 (17738129), and Arabidopsis Dicer (15223286). The secondary structure diagram is shown on the bottom. Dots denote disordered regions. Conserved residues are shaded in blue and invariant residues in red. Triangles denote residues directly interacting with RNA.