Periodicity-Atomic Radius
advertisement

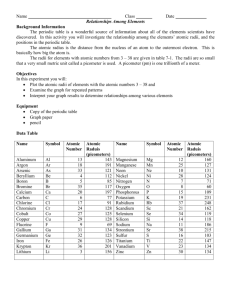
Periodicity-Atomic Radius 1. Using the chart on page 141 in your book, construct a graph of atomic radii of elements 1-36. a. Plot atomic number on the x-axis b. Plot atomic radii(pm) on the y-axis c. Give your graph a descriptive title d. Label each axis with appropriate information 2. Use a ruler to connect the plots on the graph. 3. Identify and label the elements that are located at the peaks of the graph. 4. Identify and label the elements that are located at the troughs of the graph. 5. Use your graph to answer the following questions. Write your answers in complete sentences. a. Describe the trend that exists within the PERIODS and GROUPS of the periodic table that are represented by elements 1-36.(Hint: use words like increases or decreases and give the direction of change). b. If you continue to graph the remaining elements, what pattern would you predict on your graph? Explain why. c. If you were to graph atomic number vs. ionic size, what elements would you expect to see at the peaks of your graph? d. If you were to graph atomic number vs. electronegativity, what elements would you expect to see at the peaks of your graph? e. If you were to graph atomic number vs. ionization energy, what elements would you expect to see at the peaks of your graph? 1. 2. 3. 4. 5. Periodicity-Atomic Radius Using the chart on page 141 in your book, construct a graph of atomic radii of elements 1-36. a. Plot atomic number on the x-axis b. Plot atomic radii(pm) on the y-axis c. Give your graph a descriptive title d. Label each axis with appropriate information Use a ruler to connect the plots on the graph. Identify and label the elements that are located at the peaks of the graph. Identify and label the elements that are located at the troughs of the graph. Use your graph to answer the following questions. Write your answers in complete sentences. a. Describe the trend that exists within the PERIODS and GROUPS of the periodic table that are represented by elements 1-36.(Hint: use words like increases or decreases and give the direction of change). b. If you continue to graph the remaining elements, what pattern would you predict on your graph? Explain why. c. If you were to graph atomic number vs. ionic size, what elements would you expect to see at the peaks of your graph? d. If you were to graph atomic number vs. electronegativity, what elements would you expect to see at the peaks of your graph? e. If you were to graph atomic number vs. ionization energy, what elements would you expect to see at the peaks of your graph?