Emission Lab
advertisement
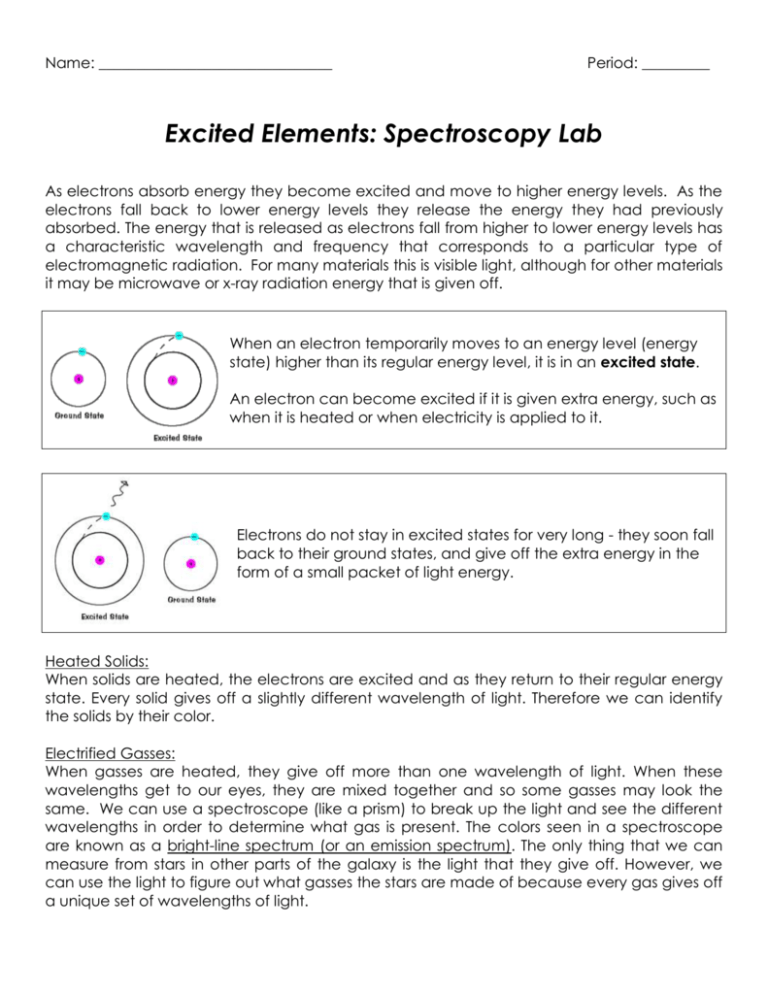
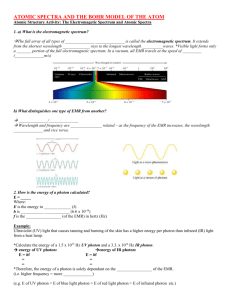
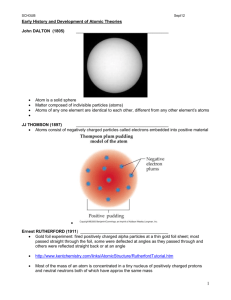
Name: _______________________________ Period: _________ Excited Elements: Spectroscopy Lab As electrons absorb energy they become excited and move to higher energy levels. As the electrons fall back to lower energy levels they release the energy they had previously absorbed. The energy that is released as electrons fall from higher to lower energy levels has a characteristic wavelength and frequency that corresponds to a particular type of electromagnetic radiation. For many materials this is visible light, although for other materials it may be microwave or x-ray radiation energy that is given off. When an electron temporarily moves to an energy level (energy state) higher than its regular energy level, it is in an excited state. An electron can become excited if it is given extra energy, such as when it is heated or when electricity is applied to it. Electrons do not stay in excited states for very long - they soon fall back to their ground states, and give off the extra energy in the form of a small packet of light energy. Heated Solids: When solids are heated, the electrons are excited and as they return to their regular energy state. Every solid gives off a slightly different wavelength of light. Therefore we can identify the solids by their color. Electrified Gasses: When gasses are heated, they give off more than one wavelength of light. When these wavelengths get to our eyes, they are mixed together and so some gasses may look the same. We can use a spectroscope (like a prism) to break up the light and see the different wavelengths in order to determine what gas is present. The colors seen in a spectroscope are known as a bright-line spectrum (or an emission spectrum). The only thing that we can measure from stars in other parts of the galaxy is the light that they give off. However, we can use the light to figure out what gasses the stars are made of because every gas gives off a unique set of wavelengths of light. Emissions Spectra Procedure: 1. Look through the spectroscope until you see BRIGHT, SHARP LINES. Fuzzy colors that show the rainbow are usually from other lights that are getting in the way. 2. Fill in the chart below. Try to color in the visible color, but if no color really fits, describe it in words and color it with the closest color possible. You should all be able to color in the spectrum. 3. When you finish coloring in the spectrum, try to match the element your spectrum corresponds with to the elements displayed on the overhead. When in doubt, ask your group members. 4. DO NOT TOUCH THE GAS SETUPS. THEY ARE DANGEROUS (AND HOT). Gas #1: ________________________ Visible color: ____________________ Gas #2: _________________________ Visible color: _____________________ Gas #3: _________________________ Visible color: _____________________ <=== BREAK FOR INTERMISSION ===> Gas #4: _________________________ Visible color: _____________________ Gas #5: _________________________ Visible color: _____________________ Gas #6: _________________________ Visible color: _____________________ During Intermission: Gas #7: Out the Window Visible color: _____________________ Gas #8: Incandescent Light Bulb Visible color: _____________________ Questions: 1. What is an “excited state” of an electron? How does an electron jump up to an excited state? 2. How does an electron in an excited state return to its ground state? 3. When there is a mixture of two gasses, the emission spectrum just looks like a combination of both spectra placed on top of each other. Take a look at your classroom’s ceiling lights through a spectroscope. Draw its emission spectrum below: a. Predict what element(s) might be in the classroom lights. _________________________ b. How did you make your prediction? 4. Which color in the spectrum has the longest wavelength? Which color in the spectrum has the shortest wavelength? 5. Higher frequencies correspond to higher energies. a. Which color in the spectrum has the highest frequency? b. Which color in the spectrum has the lowest frequency? c. Which color in the spectrum has the highest wave energy? d. Why do you think laser pointers are generally made with pure red light? 6. Take a look at the visible color you described for each element, and then take a look at the corresponding emission spectrum you drew. a. What do you think is the relationship between the emission spectrum you see and the element’s visible color? b. From your reading and your answer in part a, what do you think a spectroscope does? (What does it do to incoming light waves? Use one of your vocab terms.) 7. During the “intermission” period, you looked at the emission spectrum of the light coming in from your window and the emission spectrum of the light from an incandescent bulb. How did these spectra compare? 8. Below is a graph of Intensity versus Wavelength for an unknown element. What is this unknown element, and how did you come to that conclusion?