Spectroscopy Lab Key
advertisement

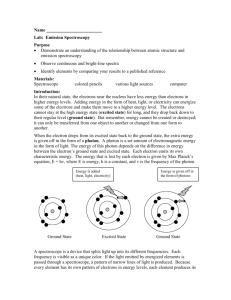
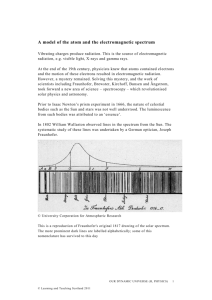
Name: Class: Date: Spectrum of Various Light (Emission Spectroscopy) Introduction Each element can be identified because it has a unique pattern of lines in its light spectrum. Niels Bohr hypothesized that electrons exist in ORBITS around each atom and each orbit has a unique ENERGY LEVEL. Bohr explained that the individual lines in an element’s spectrum are produced when atoms receive energy from an outside source (such as heat, light or electricity).These electrons move to higher energy (orbits) and quickly fall back to their original orbits. As an electron goes from a high-energy orbit to a low-energy orbit, the electron gives off its excess energy in the form of light (photon). This is the light we see when we look at an element’s spectrum. (See the diagram below.) Bohr hypothesized that because each element has a different number of electrons, it also has different energies for each of its orbits. el ectron li ght given off 1s t sh ell (1s t orb it) nu cle us 2n d sh ell (2n d orb it) In itia lly, the a tom i s “res ting qui etly” Th en a n el ectron g ets “bu mped ” up to a hi gher– energ y orbit by a bsorb ing energ y Fi nall y, the ele ctro n mo ve s ba ck to its origi nal, lo we r–ene rgy orbi t an d gi ve s off en ergy in the form of ligh t Light energy is measured by the wavelength of the sine waves produced by the light. High energy light, such as blue light, has a short wavelength (vibrates up and down quickly) while low energy light, such as red light, has a longer wavelength (vibrates up and down slowly) – see the diagram below How does a spectroscope work? A spectroscope breaks light up into the colours of the rainbow. The part of the spectroscope that actually spreads light into its various colours is called a “GRATING”. A grating is a piece of plastic that acts like a prism and is engraved with about 15,000 parallel lines per 2.5 cm. The diagram below shows how a spectroscope works. Purpose In this experiment you will use a spectroscope to observe the light given off by various light sources and use the spectroscope to analyze an unknown substance. Pre-lab Questions 1. According to Bohr’s atomic model, where may an atom’s electrons be found? The e-s are found outside of the nucleus 2. How do electrons become “excited”? With electricity, heat, and light 3. State the equation that is used to determine the energy content of a packet of light a specific frequency. E = h (Max Planks) 4. What form of energy emission accompanies the return of excited electrons to the ground state? Photons are produced (colourful light) 5. What are two sources of continuous spectra that will be use in this exercise? Incandescent (regular light bulb) and fluorescent lights 6. Why are students not permitted to touch the spectrum tube or high-voltage assembly? 40 000 volts!!! Could get a great shock... 1) Observe light from the sun, an incandescent light bulb and/or fluorescent light, as instructed by your teacher. Record what you see. It is important to draw the colours at the correct place on the numbered scale, shading one colour into the next in a continuous fashion. You MUST use coloured pencils or crayons to correctly show the ways the colours change from one to the next. 2) Observe the light from each of the following. Be sure to draw the lines exactly where you see them at the correct places on the scale. If they are not located correctly, you will not be able to answer question 5 and 6, below. Also, describe the colour of the light you see when simply looking at the light without using the spectroscope. Data and Observations Part I 1. Incandescent Light Bulb Spectrum (1 mark) Red Orange Yellow Green Blue Indigo Violet Description: (1 mark) Continuous rainbow of 7 colours 2. Fluorescent Light Spectrum Red Orange Yellow Green Blue Indigo Violet Description: (1 mark) Continuous rainbow of 7 colours with 5 additional absorption lines (thick lines) that are superimposed on the continuous spectrum (turquoise blue, lime green, ochra, red, orange) Gas tube Air Violet Indigo Blue Green Yellow Orange Red Ar CO2 Cl2 H2 He Hg I2 Kr Ne Xe unknown Discussion 1) Compare the spectra produced by incandescent and fluorescent light sources We noticed absorption lines (bright superimposed bands of light) for the fluorescent light source. 2) What are two GENERAL differences between the spectrum (or spectra: plural)? Every spectrum is different from another. A spectrum is like the fingerprint of an element. Each spectrum has different lines . 3) Two types of spectra are observed; one type is called a “line spectrum” and the other is called a “continuous spectrum”. Based on your observations, explain clearly what you think a “continuous spectrum” is and give an example of light source you observed which exhibits such a spectrum. . Continuous spectra: coloured bands are connected Ex: white incandescent light Line spectra have lines that are found in between gaps Ex: neon spectrum 4) Are ALL line spectra identical? Explain your answer. No, all substances have their own specific coloured lines. *T*5) How might the difference in the brightness of spectral lines be explained? With the bright lines, more electrons are jumping onto the same higher energy level (orbit) and returning to their ground state(lower orbit) while emitting photons (colourful lights) 6a) How did Bohr explain the way lines are formed in the light spectrum of an atom or ions? Electrons are going down to a lower orbit (energy level) while emitting a photon of colourful light. b) You used electricity to move electrons from one orbit of an atom to a higher energy orbit. What experimental evidence do you have that orbits in the different atoms have different energies? [Hint: read the introduction.] Different colours are produced due to different electrons jumping from different energy levels (orbits). 7) Scientists have accurately measured the wavelengths of every line in the spectra of all the known stable elements (some newly—discovered radioactive elements decompose too quickly to enable their spectra to be measured). What procedure could astronomer use to analyze the light coming from distant stars to decide which elements ARE present in the stars and which are NOT present. We should observe the same spectra as hydrogen and helium in the stars since nuclear fusion takes place at each star's surface. Instead, astronomers have noticed that each star's spectrum has shifted towards the red area of the rainbow. (Red shift) 8) How can the frequency of emitted light be used to determine the energy difference between orbits (energy levels)? E = h energy(E) is proportional to frequency (), h: Planck constant 9) Prior to its discovery on Earth, helium’s existence was first confirmed in the Sun. Explain how this was possible. Using a spectroscope attached to a telescope, we noticed that helium was found on the Sun's surface during a solar eclipse. The spectral lines were the same as excited helium.