Supporting Information
advertisement

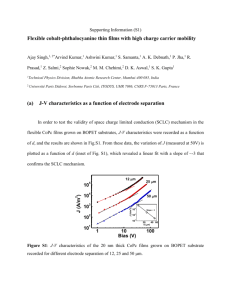
Supporting Information (S1) Bending stress induced improved chemi-resistive gas sensing characteristics of flexible cobalt-phthalocyanine thin films Ajay Singh1, 2*,#, Ashwini Kumar1, Arvind Kumar1, S. Samanta1, Nirav Joshi1, A. K. Debnath1, R. Prasad1, Z. Salmi2, M. M. Chehimi*2,#, D. K. Aswal*1,#, S. K. Gupta1 1 Technical Physics Division, Bhabha Atomic Research Center, Mumbai-400 085, India 2 Université Paris Diderot, Sorbonne Paris Cité, ITODYS, UMR 7086, CNRS F-75013 Paris, France (a) Experimental details The charge transport measurements were carried out using in-plane electrode geometry. After cobalt phthalocyanine (CoPc) film deposition, few pairs of planar gold electrodes (size: 3mm x 2mm) separated by 12 m were thermally evaporated onto films using a metal mask. Subsequently, electrical contacts were taken using silver wires attached to the gold electrodes using silver paint. The bending measurements were performed by attaching the flexible samples to curved surfaces of different radius. The current-voltage (J-V) measurements were carried out using Keithley 6487 voltage-source / pcoammeter and computer based data acquisition system. XPS spectra were obtained using a Thermo VG ESCALAB 250 instrument fitted with a monochromated Al-Kα X-ray source. For gas sensing experiments the response curves (resistance versus time data) were recorded using a static gas testing setup. Briefly, the sensor was mounted in a leak tight stainless steel chamber (net volume: 1000 cm3). A desired concentration of the test gas in the chamber was achieved by injecting a known quantity of gas using a micro-syringe. The response data was acquired by a personal computer equipped with 1 Labview software. Once a steady state was achieved, recovery of films was recorded by exposing the sensors to air by slight opening of the lid of chamber. (b) XPS spectra of high mobility flexible CoPc films Figure S1 shows the survey X-ray photoelectron spectroscopy (XPS) spectra of the CoPc films grown on bi-axially oriented polyethylene terephthalate (BOPET) substrate, which shows the presence of only C1s, N1s, Co2p, CoLVV auger and O1s signal in the spectrum. Figure S1: Survey XPS spectra for the CoPc films 20 nm thick CoPc films grown on BOPET substrate. Figure S2 shows the high resolution XPS spectra of C1s, N1s, Co2p, and O1s for the CoPc films grown on BOPET. The spectra can be described analogous to other metal phthalocyanines.1 2 (a) C 1s I (arb. units) I (arb. units) 282 285 288 291 398 B. E. (eV) Co 2p Co-2p3/2 Co-2p1/2 780 790 800 400 B. E. (eV) 402 O 1s (d) I (arb. units) (c) I (arb. units) N 1s (b) 810 530 532 534 536 538 B. E. (eV) B. E. (eV) Fig. S2: XPS spectra for the CoPc films (a) C1s (b) N1s(c) Co2p and (d) O1s spectra. The C1s peak consists of three main components which are quite clearly distinguished. The features at binding energies of 284.9 eV and 286.2 eV are attributed to aromatic carbon of the benzene rings and to pyrrole carbon linked to nitrogen, respectively. The lower intensity components (at 288.2 eV) is attributed to π-π* satellites. As for other Pcs, the N1s spectra (Fig 2(b)) consist of two peaks centered at about 399.6 eV and 401.4 eV respectively representing the two chemically slightly different N atoms in CoPc. One of the N atoms (399.6 eV) corresponds to the pyrrole ring without any coordination to the Co atoms and other peak corresponds to the N atom (401.4 eV) which are also bonded to the centre Co atoms. The XPS spectra displayed in Fig. 2(c) represent the Co2p3/2 and Co2p1/2 signal of CoPc films at 781.8 eV and 797.2 eV 3 respectively. By taking area under curve for C1s, N1s and Co2p XPS peaks and corresponding elemental sensitivity factors, the atomic ratio C/Co and N/Co was found to be 31.7 and 7.6 respectively. It indicates the films have same chemical composition (C32H16CoN8) as that of source CoPc material. Interestingly CoPc films also exhibit the presence of chemisorbed oxygen (at 533.8 eV) over the sample surface (shown in Fig. S2(d)). The XPS signal of O-1s can come from BOPET ((C10H8O4)n) substrate also, for which C/O ratio is ~ 2.5. For the CoPc films the atomic ratio of C/O was found to be 33, which indicates the detected oxygen is a surface adsorbed oxygen. It may be noted that due to low coordinated structure of Co atoms in CoPc molecules, it is the most preferable sites for the chemisorptions of oxygen. (b) Current-voltage (J-V) and chemi-resistive gas sensing characteristics of CoPc films grown at low substrate temperature In order to show the importance of structural ordering (hence of mobility) for gas sensing characteristics of CoPc films, the films were prepared with poor structural ordering on BOPET substrate. Structural defects were introduced by growing the films at lower substrate temperature (~ 50ºC).The J-V characteristics of such poorly ordered CoPc films is shown in Fig. S3 (a). From the comparison of J-V characteristic of high mobility (shown in Fig. 1 (c)) films with the J-V data of these films, it can be seen that (i) current (at a particular bias for ex. at 50V) for these low temperature grown CoPc films is nearly two orders of magnitude lower and (ii) J-V characteristic shows hysteresis. The low current and hysteretic J-V characteristics is consequence of poor structural ordering in the films due to their synthesis at low substrate temperature.2 The analysis of J-V curves gives the estimated values of μ ~ 9.4 cm2/V-s. The atomic force microscope (AFM) image shown in the inset of Fig. S3 (a), also confirms the presence of many grain boundaries hence poor structural quality of these samples. 4 0 1.0µm 5 -1x10 5 -2x10 2.4 30 15 0 20 30 40 50 Concentration (ppm) 2.0 50 ppm 8 1 μm (b) 45 30 ppm 5 2.8 Sensitivity 1x10 (a) 20 ppm 5 R (10 ) 2 J (A/m ) 2x10 1.6 -90 -60 -30 0 30 Bias (V) 60 0 90 2000 4000 6000 Time (sec) 8000 Fig. S3: (a) J-V characteristics for the CoPc films deposited at substrate temperature of 50ºC. Inset shows the AFM images of the films. (b) Response curve (resistance versus time) of low mobility CoPc films for two different concentration of NH3. Red dotted line is the guide to see the starting base resistance of the films. Response curve recorded for the poorly ordered CoPc films for different NH3 concentration is shown in Fig S3(b). It can be seen that the presence of defect states in these films results in many inferior gas sensing properties (as compared to the high mobility CoPc films) such as minimum detection limit (MDL) of these films is 20 ppm, low sensitivity, sluggish response / recovery and base resistance drift after each exposure. References 1. F. Petraki, H. Peisert, I. Biswas. T. Chasse, J. Phys. Chem. C 114, 17638 (2010). 2. A. Singh, S. Samanta, A. Kumar, A. K. Debnath, D. K. Aswal, S. K. Gupta, J. V. Yakhmi, Y. Hayakawa, Org. Elec. 11, 1835 (2010) 5