Trends in the Periodic Table I: Atomic and Ionic Radius
advertisement

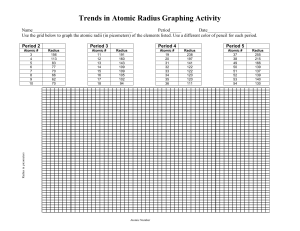
Jens-Uwe Kuhn, Santa Barbara City College Jessica Martin, Northeastern State University Creative Commons License: Attribution-Noncommercial-Share Alike 3.0 United States License. Trends in the Periodic Table I: Atomic and Ionic Radius Many of the trends in the periodic table are useful tools for predicting electronic properties and chemical reactivities of various species, including transition metal complexes. The radius of transition metal ions in inorganic coordination compounds is of great importance in many biologically relevant coordination compounds. Objectives: (1) Predict the variation in atomic radius in the periodic table. (2) Describe specific reasons for this variation across a period and within a group. (3) Predict differences in atomic radius and ionic radius for the same element; predict differences in ionic radius for various oxidation states of the same element. A. Atomic Radius Critical Thinking Questions: 1. Write out the complete electron configuration for lithium, sodium, and potassium. 2. Write out the complete electron configuration for beryllium, magnesium, and calcium. 3. How do the valence electron configurations change as you go from one element to the next in question 1? How do the valence electron configurations change as you go from one element to the next in question 2? How do the complete electron configurations differ from one another for the elements in each of the above questions? 4. Predict the trend for the atomic radius for the elements in group I and group II of the periodic table. 1 5. Compare your predictions to the values given in model 1 below. Model 1: Atomic radii in groups I and II of the periodic table: Element Lithium Sodium Potassium Rubidium Cesium Beryllium Magnesium Calcium Strontium Barium 1 Atomic Radius (pm)1 145 180 220 235 260 105 150 180 200 215 Slater, J.C., J. Chem. Phys., 1964, 41, 3199. 6. Write out the complete electron configuration of titanium, and the valence electron configurations of vanadium, chromium, iron, cobalt, arsenic, and selenium. Keep in mind that the electron configuration of some atoms differs from what may be expected. 7. How do the complete and/or valence electron configurations change as you go from one element to the next in question 6? 8. Going from left to right in period 4 of the periodic table (or any other period), what do you predict the general trend to be for the atomic radius of the elements? Consider the total number of protons and electrons, as well as the valence shell that electrons are added into when going from left to right across a period in your prediction. 2 9. Compare your predictions to the values given in model 2 below. Model 2: Atomic radii in period 4 of the periodic table: Element Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine 1 Atomic Radius (pm)1 220 180 160 140 135 140 140 140 135 135 135 135 130 125 115 115 115 Slater, J.C., J. Chem. Phys., 1964, 41, 3199. 10. Are there any exceptions to the general trends for the atomic radius that can be seen in these models? If so, suggest an explanation for these exceptions. B. Ionic Radius Critical Thinking Questions: 11. In general, will an anion have a larger or smaller ionic radius compared to the atomic radius of the neutral element that it is derived from? Justify your answer. 3 12. Is the same true for cations? Justify your answer. Problems: 13. Considering your answer to the previous question, use the information given in model 2 to predict the ionic radius for Fe2+, Fe3+, V4+, and V5+. 14. Compare your predicted ionic radii from the previous question to values given in the literature. 15. Order the ions listed in question 13, as well as V2+ and V4+, in order of increasing size to charge ratio. 16. Discuss at least two possible effects that a different size to charge ratio of transition metal ions derived from the same element (e.g. Fe2+ and Fe3+) could have on properties, reactivities, or chemical behavior of compounds containing inorganic transition metal ions. 4