Rutherford observed a nuclear reaction in 1919
advertisement

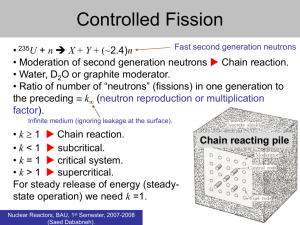
Chapter 31 Nuclear Energy Rutherford observed a nuclear reaction in 1919. This was transmutation, changing one formerly immutable element into another. A typical reaction fires particles at nitrogen nuclei, changing 4 14 17 1 14 17 the nitrogen to oxygen. We write He N 0 H or N(, p) 0 . 2 7 8 1 7 8 Note that both electric charge and nucleon numbers Z and A are conserved. Both energy and momentum are conserved. Define Q = M M N M p M O c 2 KE KE N KE p KE 0 . If Q > 0, the reaction is exothermic or exoergic and releases energy. Fermi used neutrons to bombard targets and due to the lack of electrical repulsion between the target and the neutral particles was unable to produce new transuranic elements. Fission In 1928 Hahn and Strassmann found n 235 236 141 92 U U Ba Kr 3n . 92 92 56 36 This was explained by Meitner and Frisch as a fission of the uranium nucleus. Usually the nucleus splits unequally. Fission releases energy as the mass of the original nucleus exceeds that of the fragments. From the curve of binding energy per nucleon, we find about 200MeV released per fission. (7.6MeV /nucleon for 235 nucleons in U compared to 8.5 MeV/nucleon in the fragments). Fermi created a fission chain reaction in 1942. The neutrons emitted in fission are fast. If they can be slowed by collision with a moderator, they can induce other atoms to fission. The moderator is most effective if its mass is about the same as that of the neutron. Regular 2 hydrogen absorbs neutrons, however, so a good choice is deuterium H. Fermi used graphite 1 12 C for its easy availability and structural properties. 6 The fuel in the reactor is enriched (a greater than normal percentage of common 235 U mixed with 92 238 U) to account for some neutron absorption. 92 There must be a critical mass of fuel so neutrons don't escape before causing further fissions. This usually means a few kg of highly enriched uranium. The multiplication factor f tells how many neutrons from each fission go on to cause another fission. Keeping a reactor critical demands f 1. About 1% of the neutrons emitted in fission are delayed instead of prompt neutrons. This makes it possible to control the reactor by use of control rods made of cadmium or boron. These materials absorb neutrons. The few seconds afforded by delayed neutrons is just enough time to insert or withdraw rods to bring a reactor to criticality. Power reactors are used in many areas to generate electricity as the heat from the reactions turn water into steam. The heat comes from kinetic energy given to fuel and moderate in the core. In 235 the US, fuel is enriched to 2% - 4% U. There are no greenhouse gases from this power 92 source, but disposal of the radioactive waste is a problem. Reactors cannot explode like a bomb. Research reactors (one is at Purdue) are smaller than power reactors. The atomic bomb, first exploded in 1945, drives two sub-critical masses of 235U or 239PU together. With f>1, an explosion results that destroys a city. Fusion In this nuclear reaction, light elements are fused together, releasing energy like the stars. For 1 4 example, 4 H He 2e t 2 2 (through a series of reactions); this powers the sun. 1 2 The hydrogen or thermonuclear bomb uses high temperatures of 108K from a fission bomb to trigger fusion. This energy is needed to overcome electrical repulsion and fuse protons together. These bombs can be 500 times as powerful as an atomic bomb. We cannot yet control fusion in a reactor. Magnetic confinement and inertial confinement approaches are still being studied, but after fifty years of research, this ultimate energy source eludes us. It would produce little radioactive waste and we have an unlimited amount of fuel. Passage of Radiation Through Matter When or particles pass through matter these charged particles (called ionizing radiation) attract or repel electrons. Each or can remove thousands of electrons, chemically altering material. X-rays and -rays also damage material by ejecting electrons by the photoelectric effect and Compton effect. Collisions from neutrons also cause damage. Dosimetry 1 radioactive decay per second (in SI units) is the bequerel. 1 Bq = 1 decay/s. 2 N .693 N N is the activity of a radioactive source. This can be measured t T1 / 2 in Bq or curies where 1 curie (Ci) = 3.7 x 1010 disintegrations/second. This is the radioactivity of 1 gram of radium. Recall activity The absorbed dose of radiation can be measured in roentgens (R); 1 roentgen of X-rays deposits .878 x 10-2J/kg of energy in air. The rad is the amount of radiation depositing 0.01J/kg in any absorber. Today we use (in SI units) the gray (Gy) where 1 Gy = 1J/kg deposited radiation = 100 rad. Now 1 rad of radiation does twenty times the damage of 1 rad of radiation; the effects also depend on what type of material the radiation is passing through. We define the effective dose in rem = dose in rad times QF (quality factor) to account for or or . . . ). The preferred SI unit is the sievert; effective dose in sieverts (Sv) = dose in grays (Gy) times QF. 1 rem or sievert of any type of radiation does the same damage. There is a natural background radiation. Radon gas seeps into our houses through the foundation in this area (it is formed as part of the uranium decay chain). Radar undergoes decay and its decay products can also interact with the body. Generally limiting our exposure to less than 0.5 rem/year from all natural and artificial sources is considered safe. It takes a 400-1000 rem dose delivered in a short time to kill a person. Smaller doses increase the risk of cancer. Radiation can be beneficial. We will have local medical professionals speak about radiation therapy, X-rays, CAT scans, MRI, tracers and other medical applications. 3