13) According to the Bohr atomic model, why do
advertisement

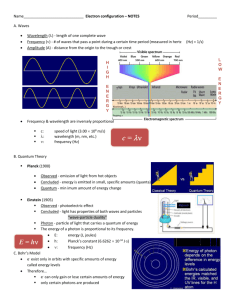
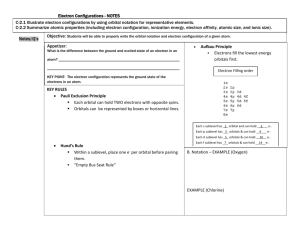
13) According to the Bohr atomic model, why do atomic emission spectra contain only certain frequencies of light? Because only certain atomic energies are possible, only certain frequencies of radiation can be emitted from an atom. 14) Why is the wavelength of a moving soccer ball not detectable to the naked eye? It is too small to see or detect 15) What sublevels are contained in the hydrogen atom’s first four energy levels? What orbitals are related to each s sublevel and each p sublevel? Energy level 1 2 3 4 Sublevel s s, p s, p, d s, p, d, f Each s sublevel is related to a spherical s orbital. Each p sublevel is related to three dumbbell-shaped orbitals (px, py, and pz). 16) Use de Broglie’s wave-particle duality and the Heisenberg uncertainty principle to explain why the location of an electron is an atom is uncertain. An electron has wave-particle characteristics and does not have a single, definite location in space. The Heisenberg uncertainty principle states that it is fundamentally impossible to know precisely both the velocity and position of a particle at the same time. 17) Compare and contrast the Bohr model and quantum mechanical model of the atom. Bohr model: the electron is a particle; the hydrogen atom has only certain allowable energy states. Quantum mechanical model: the electron is a wave-particle phenomenon; an electron’s energy is limited to certain values. Also, the quantum mechanical model makes no assertions regarding the electron’s path around the nucleus.