AS_Unit1_Particle_01_Atomic_Structure_Answers
advertisement
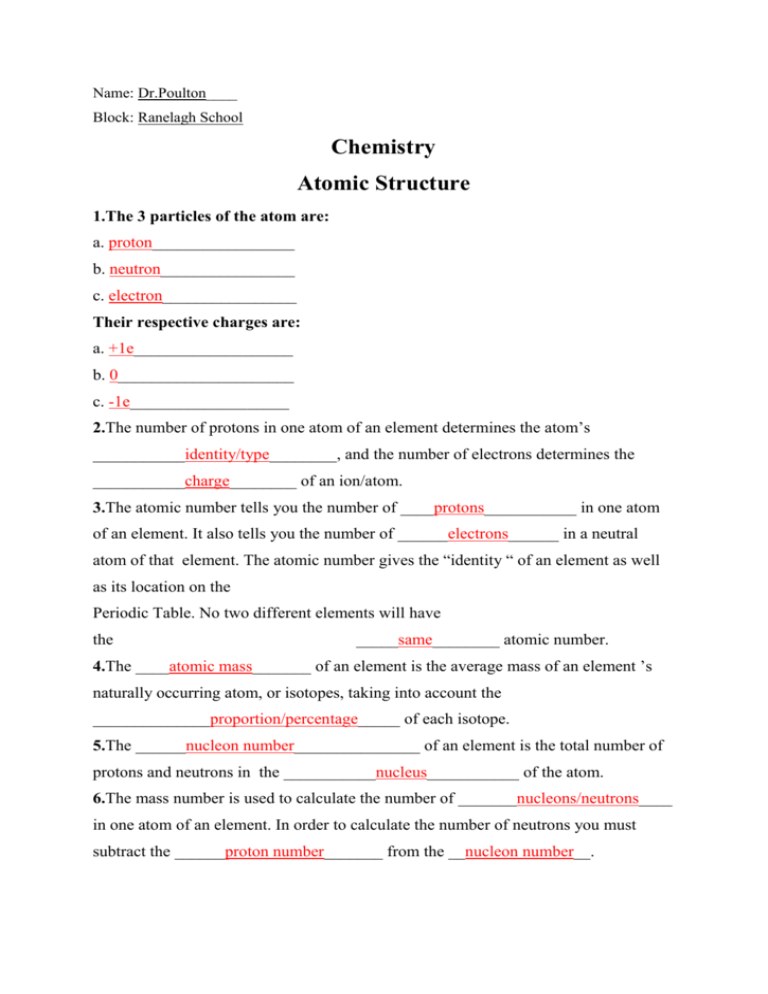
Name: Dr.Poulton____ Block: Ranelagh School Chemistry Atomic Structure 1.The 3 particles of the atom are: a. proton_________________ b. neutron________________ c. electron________________ Their respective charges are: a. +1e___________________ b. 0_____________________ c. -1e___________________ 2.The number of protons in one atom of an element determines the atom’s ___________identity/type________, and the number of electrons determines the ___________charge________ of an ion/atom. 3.The atomic number tells you the number of ____protons___________ in one atom of an element. It also tells you the number of ______electrons______ in a neutral atom of that element. The atomic number gives the “identity “ of an element as well as its location on the Periodic Table. No two different elements will have the _____same________ atomic number. 4.The ____atomic mass_______ of an element is the average mass of an element ’s naturally occurring atom, or isotopes, taking into account the ______________proportion/percentage_____ of each isotope. 5.The ______nucleon number_______________ of an element is the total number of protons and neutrons in the ___________nucleus___________ of the atom. 6.The mass number is used to calculate the number of _______nucleons/neutrons____ in one atom of an element. In order to calculate the number of neutrons you must subtract the ______proton number_______ from the __nucleon number__. 7.Give the symbol and number of protons in one atom of: Lithium _____Li, 3______________ Bromine _____Br, 35____________ Iron ________Fe, 26_____________ Copper ______Cu, 29____________ Oxygen _____O, 8_______________ Mercury _____Hg, 80____________ Krypton ____ Kr, 36_____________ Helium ______He, 2_____________ 8.Give the symbol and number of electrons in a neutral atom of: Uranium _____U, 92____________ Chlorine ___Cl, 17_____________ Boron _______B, 5_____________ Iodine _____I, 53_______________ Antimony ____Sb, 51___________ Xenon _____Xe, 54_____________ 9.Give the symbol and number of neutrons in one atom of: (To get “mass number ”,you must round the “atomic mass ” to the nearest whole number)) Show your calculations. Barium: Ba, N = A – Z = 137 – 56 = 81 Bismuth: Carbon: C, N = A – Z = 12 – 6 = 6 Fluorine: F, N = A – Z = 19 – 9 = 10 Bi, N = A – Z =_209 - 83 =126 Hydrogen: H, N = A – Z = 1 – 1 = 0 Magnesium: Mg, N = A – Z = 24 – 12 = 12 Europium: Eu, N = A – Z = 152 – 63 = 89 Mercury: Hg, N = A – Z = 201 – 80 = 121 10.Name the element which has the following numbers of particles: a.26 electrons,29 neutrons,26 protons ___________________Iron_______ b.53 protons,74 neutrons _____________________________Iodine________ c.2 electrons (neutral atoms)___________________________Helium______ d.20 protons _______________________________________Calcium______ e.86 electrons,125 neutrons,82 protons (charged atom) _____Lead (4- ion)_____ f.0 neutrons _______________________________________Hydrogen_____ 11 .If you know only the following information can you always determine what the element is? (Yes/No). a. number of protons _____________________Yes___ b. number of neutrons ____________________No____ c. number of electrons in a neutral atom _____Yes____ d. number of electrons ___________________No_____