LAB 4: HEAT OF FUSION
advertisement

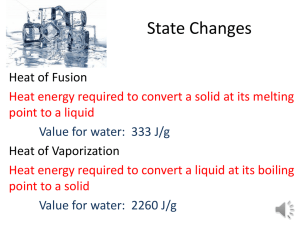
LAB 4: HEAT OF FUSION Learning Objectives 1. Measure the Heat of Fusion of water. 2. Compare the experimentally measured Heat of Fusion with the accepted standard and compute the percentage difference. 3. Learn the principles of design and operation of a simple calorimeter. 4. Learn to read a laboratory thermometer to 0.1oC by estimating between the lines of the thermometer graduations, or by directly reading a digital electronic thermometer. 5. Increase the accuracy of a laboratory measurement by calculating the average of three different measurements. 6. Learn the Specific Heat relationship between amount of heat absorbed during fusion of ice and the mass of the ice. 7. Utilize the Specific Heat of water by using the relationship between the amount of heat gained by by a given amount of water and the temperature increase of the water. 8. Utiilize the principle of heat transfer in an insulated calorimeter that the heat absorbed by the ice during fusion will be equal to the heat lost by the water. Background Scientific Principles Heat of Fusion Changes of state (phase changes) involve the conversion or transition of matter from one of the common states (solid, liquid or gas) to another. Examples include fusion or melting (change from the solid to the liquid state), freezing (change from the liquid to the solid state), evaporation or vaporization (change from the liquid to the gaseous state), condensation (change from the gaseous state to the liquid state), sublimation (change from the solid directly to the gaseous state), and deposition (change from the gaseous state directly to the solid state). Heat of Fusion is the amount of heat released as 1 kilogram of liquid freezes (solidifies or crystallizes), or the amount of heat absorbed as a solid melts (fuses or liquefies). See your textbook, p. 115-116. The exact value of the Heat of Fusion is different for different substances. For water, the Heat of Fusion is 335 kiloJoules per kilogram, or 335 Joules per gram, also expressed as 80 calories per gram. Although the Joule is the official SI unit for measurement of energy, it will be simpler to calculate in this experiment if we use the calorie as an energy unit. 1 calorie = 4.184 Joules. The calorie is simple to use in problems involving water, because 1 calorie of heat energy will raise the temperature of 1 gram of water by 1oC. Typically, changes of state of a pure substance, such as melting, freezing, evaporation, condensation, etc., occur at a constant temperature. Temperature change only occurs when all of the phase changes are completed. This means that during the phase change, energy is being absorbed or emitted with no change in temperature of the material being studied. This energy can be absorbed or supplied by some other substance in the environment, and absorbing or supplying that energy can alter the temperature of that material. Specific Heat When a substance changes temperature, but does not change its state, its temperature goes up by an amount proportional to the amount of heat energy absorbed or lost. The specific amount of energy that must be lost or gained to change the temperature of a standard mass (1 kilogram for physics) of a substance by 1o Celsius is called the Specific Heat. The exact value of the specific heat varies, depending on the particular substance. For water, the Specific Heat is 4.2 kiloJoules per kilogram per o Celsius, or 4.2 joules per gram per o Celsius, also expressed as 1.0 calories per gram per oC. Application of Heat of Fusion and Specific Heat in this experiment In our study today, we are going to study the Heat of Fusion of water. We are going to place some solid water (ice) at its melting temperature of ~0oC into some warm liquid water that has been heated to a warmer temperature than the lab room. By doing this procedure in an insulated container called a calorimeter, we will limit transfer of heat to between the ice and the water. Because of the insulation, very little heat will be transferred between the water or ice and the environment. The calorimeter simply consists of a small styrofoam cup within a larger styrofoam cup. An insulating cardboard or styrofoam cover is pierced by a hole to allow insertion of a thermometer, which also serves as a stirring device. The insulation (styrofoam,cardboard and air spaces) of the calorimeter will prevent heat transfer to any other substance or material in the environment. By assuming that the heat absorbed by the styrofoam calorimeter and air is approximately zero, we can approximate the heat absorbed by the ice as it melts as being equal to the heat lost by the water as it cools off. Heat gained = Heat lost equation 1 The heat gained by the ice as it melts is calculated by multiplying the mass of the ice times the heat of fusion of ice. (See Text, p. 115.) Heat gained (ice) = mice Hf equation 2 Once the ice melts, the cold water from the melted ice (which will be at the same temperatue as the ice) will gain some heat from the warm water in the calorimeter. The heat gained by the cold water is calculated by multiplying the mass of the ice times the specific heat of water times the rise in temperature of the cold water. (See Text, p. 97.) Heat gained (ice water) = mice Hsp (Tice – Tfinal) equation 3 The total heat gained is equal to the sum of equations 2 and 3. Total Heat gained = mice Hf + mice Hsp (Tice – Tfinal) equation 4 The heat lost by the warm water in the calorimeter will be calculated by multiplying the mass of the warm water times the specific heat of water times the drop in temperature. Heat lost (warm water) = mw Hsp (Tw – Tfinal) equation 5 The total heat lost is equal to equation 4. Since heat lost equals heat gained (equation 1), then equation 5 equals equation 4. mice Hf + mice Hsp(Tice – Tfinal) = mw Hsp (Tw – Tfinal) equation 6 Equation 6 can be solved for the Heat of Fusion. mw Hsp (Tw – Tfinal) – mice Hsp (Tice – Tfinal) Hf = ___________________________________________ mice equation 7 Value and use of the average of several measurements Scientists have long known by experience, and statisticians have proved mathematically, that the average of several measurements is more likely to be closely representative of the actual value than is any one of the individual measurements. During the process of making several measurements, we can also evaluate if any of our measurements deviate seriously from the others. Such deviant values may reflect serious experimental errors in either our measurements, calculations or procedures. Deviant measurements, called outliers, are usually discarded, and the measurements are repeated until the required number of reasonable values have been obtained. For our purposes, we will use the the standard that any value of Hf that deviates by more than 20% from the others is an outlier, and will be discarded*. Materials Calorimeter: larger and smaller styrofoam cups cardboard or styrofoam cover Thermometer 400 mL Beaker Laboratory balance Ice Distilled water Hot plate Paper towels Please note, if the Physical Science thermometers measure temperature only in degrees Fahrenheit (oF), the temperatures measured must be converted to oC, by using the formula C = (oF + 32) ÷ 1.8 o _____________________________________________________________________________ *The Science of Statistics, as well as the Art of Accounting have often been characterized by the aphorism “Figures lie and liars figure.” Experimental Procedure 1. Determine the room temperature by setting the thermometer on the table for 5 minutes, and then reading it. Record the temperature in oF, as it is displayed, then calculate and record the temperature in oC to the same number of decimal places as the thermometer reading. 2. Fill the 400 ml beaker about half full of distilled water from the special supply bottle. 3. Place the beaker of water on the hot plate and heat to a temperature about 15oC (27oF) above room temperature. If the water becomes too hot, you can cool it down by adding room temperature distilled water from the supply bottle. 4. Assemble the inner and outer calorimeter cups and put on the cardboard cover. 5. Weigh the assembled calorimeter, and record the weight. 6. Fill the inner calorimeter cup about half full of warm water from the beaker on the hot plate. 7. Re-cover the calorimeter and weigh the combined cup and the water, and record the mass. 8. Insert the thermometer into the ice container. 9. Allow the temperature to stabilize, a few minutes, and then read the thermometer, recording the temperature as Tice. 10. Insert the thermometer into the calorimeter, and stir briefly. 11. When the temperature stabilizes, read the thermometer, estimating between the lines to the nearest 0.1oC, recording the temperature of the warm water as Tw. 12. Acquire a tablespoon or so of ice, dry the pieces of ice with paper towels, and dump the dried ice into the calorimeter. Be careful not to touch the ice with your bare fingers or hands, as this will cause melting and create errors in your results. 13. Quickly cover the calorimeter and stir with the thermometer, until all the ice has melted. If necessary, add more dried ice until the temperature reaches at least 10oC below room temperature. 14. When all the ice has melted, and the temperature reaches a low point, around 5-10oC (40-50oF), record the temperature, as Tfinal. 15. Remove the thermometer and weigh the covered calorimeter and cool water. Record the mass. 16. Subtract the mass of the calorimeter from the mass of the calorimeter and warm water. Record as the mass of the warm water, mw. 17. Subtract the mass of the calorimeter and warm water from the mass of the calorimeter and cold water. Record as the mass of the ice, mice. 18. Use equation 7 to calculate the Heat of Fusion of water. mw Hsp (Tw – Tfinal) – mice Hsp (Tice – Tfinal) Hf = _____________________________________________ mice equation 7 Use the value of 1.0 calories per gram per oC for the Hsp of water. 19. Repeat steps 6, 7, and 11 through 18 until you obtain 3 non-deviant values for Hf. 20. Add the three values of Hf to obtain a total. 21. Divide the total obtained in step 20 by three to obtain the average. 22. Subtract the average experimental value of Hf for water, obtained in step 21, from the accepted standard value of 80.0 calories per gram, divide by the standard and multiply times 100 to obtain the per cent difference between your value and the standard. Standard – Experimental 80.0 – Average Per Cent Difference = ______________________ 100% = _______________ 100% Standard 80.0 Be sure and mention this per cent difference in the Results and Discussion sections of your lab report. 23. In the Discussion section of your lab report, state your conclusion by answering the following question: Did your experimental determination of the Heat of Fusion of water agree with, or come close to the accepted standard value? Explain why or why not.