Teacher Notes - Juniata College
advertisement

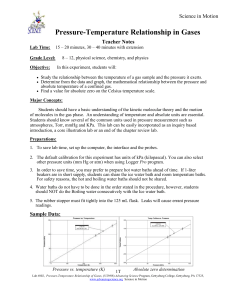
Science in Motion Boyle’s Law: Pressure-Volume Relationship in Gases Teacher Notes Lab Time: Grade Level: Objective: 15 – 20 minutes, 30 – 40 minutes with extension 8 – 12, physical science, chemistry, and physics In this experiment, students will: Use a Gas Pressure Sensor and a gas syringe to measure the pressure of an air sample at several different volumes. Determine the relationship between pressure and volume of the gas. Describe the relationship between gas pressure and volume in a mathematical equation. Use the results to predict the pressure at other volumes. Major Concepts: Students should have a basic understanding of the kinetic molecular theory and the motion of molecules in the gas phase. An understanding of temperature and absolute units are essential. Students should know several of the common units used in pressure measurement such as atmospheres, Torr, mmHg and KPa. This lab can be easily incorporated as a inquiry based introduction, a core illustration lab or an end of the chapter review. Preparations: Only preparation is the set up of computer, interface and probe. 1. This experiment is written for two types of pressure sensors, the original Pressure Sensor, and the newer Gas Pressure Sensor. The default calibration for this experiment has units of kPa (kilopascal). You can also select other pressure units (mm Hg or atm) when using Logger Pro program. 2. In order to save time, you may prefer to do Step 1 of the student procedure prior to the start of class. 3. One error in this experiment is the small inside volume of the white stem that leads to the inside of the Gas Pressure Sensor. The volume of this space is about 0.8 mL. This means that when students enter a volume of 10 mL (as read on the syringe), the volume is really about 10.8 mL. To compensate for this error, you can have your students add 0.8 mL to each of the volumes they enter. They will get better results for the value of the exponent, b, in Step 6. Boyles Law: Pressure – Volume Relationship of Gase was adapted from (Lab #6) Chemistry with Computers, by Dan Holmquist and Donald Volz. The lab was adapted by Jack Sipe for the Advancing Science Program at Gettysburg College, Gettysburg, PA 17325 http://www.advancingscience.org 1T Lab #801, Boyles Law: Pressure-Volume Relationship of Gases, (5/28/08) Advancing Science Program, Gettysburg College, Gettysburg, PA 17325, www.advancingscience.org Science in Motion Teacher Notes Science in Motion Sample Data: Pressure vs. Volume Pressure vs. Reciprocal of Volume Volume (mL) Pressure (kPa) Constant, k (P•V) 5.0 204.6 1023 7.5 136.8 1026 10.0 103.3 1033 12.5 82.1 1026 15.0 69.9 1048 17.5 58.8 1028 20.0 50.7 1013 Answers to Questions: 1. If the volume is doubled from 5.0 mL to 10.0 mL, what does your data show happens to the pressure? Show the pressure values in your answer. When the volume was doubled, the pressure was halved (pressure went from 204.6 kPa to 103.3 kPa). 2. If the volume is halved from 20.0 mL to 10.0 mL, what does your data show happens to the pressure? Show the pressure values in your answer. When the volume was halved, the pressure doubled (pressure went from 50.7 kPa to 103.3 kPa). 3. If the volume is tripled from 5.0 mL to 15.0 mL, what does your data show happened to the pressure? Show the pressure values in your answer. The pressure is reduced by a factor of 1/3 (pressure went from 204.6 kPa to 69.9 kPa). 2T Lab #801, Boyles Law: Pressure-Volume Relationship of Gases, (5/28/08) Advancing Science Program, Gettysburg College, Gettysburg, PA 17325, www.advancingscience.org Science in Motion Teacher Notes Science in Motion 4. From your answers to the first three questions and the shape of the curve in the plot of pressure vs. volume, do you think the relationship between the pressure and volume of a confined gas is direct or inverse? Explain your answer. From the data, there appears to be an inverse relationship. When pressure increases, volume seems to decrease proportionally. The shape of the pressure-volume plot appears to be a simple inverse relationship. 5. Based on your data, what would you expect the pressure to be if the volume of the syringe was increased to 40.0 mL? Explain or show work to support your answer. If the volume is increased to 40.0 mL, one would expect the pressure to be 1/2 of what it was at 20.0 mL. This would be a pressure of approximately 25 kPa. 6. Based on your data, what would you expect the pressure to be if the volume of the syringe was decreased to 2.5 mL? Explain or show work to support your answer. 7. 8. 9. 10. If the volume were reduced to 2.5 mL, one would expect the pressure to be double what it was at 5.0 mL. This would be a pressure of approximately 400 kPa. What experimental factors are assumed to be constant in this experiment? The temperature and the number of molecules in the gas sample are assumed to be constant. One way to determine if a relationship is inverse or direct is to find a proportionality constant, k, from the data. If this relationship is direct, k = P/V. If it is inverse, k = P•V. Based on your answer to Question 4, choose one of these formulas and calculate k for the seven ordered pairs in your data table (divide or multiply the P and V values). Show the answers in the third and fourth columns of the Data and Calculations table. The correct formula for an inverse relationship is: k = P•V. For k values, see the third column of the sample results on this page (1028 is the average value for the constant, k). How constant were the values for k you obtained in Question 8? Good data may show some minor variation, but the values for k should be relatively constant. Values were quite constant, with a very small deviation. Using P, V, and k, write an equation representing Boyle’s law. Write a verbal statement that correctly expresses Boyle’s law. The equation representing Boyle’s law is: k = P•V. The pressure of a confined gas varies inversely with the volume of the gas if the temperature of the sample remains constant. 3T Lab #801, Boyles Law: Pressure-Volume Relationship of Gases, (5/28/08) Advancing Science Program, Gettysburg College, Gettysburg, PA 17325, www.advancingscience.org Science in Motion