3 Vapour Pressure
advertisement
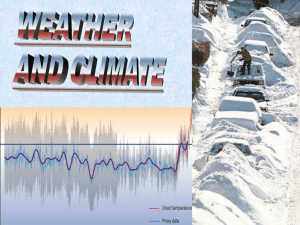
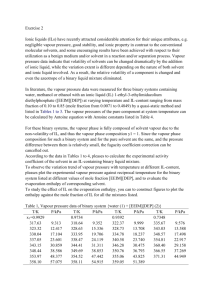
Vapour Pressure - All volatile liquids spontaneously evaporate. When they do the vapour produces a pressure. This pressure depends on temperature. The higher the temperature, the greater the rate of evaporation, the greater the vapour pressure. Vapour pressure depends on the attractive forces between particles. The greater the force of attraction the lower the vapour pressure. Alcohol has a greater vapour pressure than water because the attractive forces between alcohol molecules are weaker than between water molecules. When gases are collected over water there is a mixture of water vapour and the gas being collected. Both gases together have a pressure equal and opposite to air pressure. Sample Problem - If Carbon dioxide gas is collected at 21oC what is the pressure of the gas alone if the air pressure is 102.24 kPa? At 21oC Vapour pressure of water is 2.49 kPa 102.24 kPa = 2.49 kPa + PCO2 PCO2 = 102.24 kPa - 2.49 kPa = 99.75 kPa If hydrogen gas is collected over water at 12oC and air pressure is 98.2 kPa what is the pressure of the hydrogen gas alone? If carbon dioxide gas is collected over water at 17oC and air pressure is 99.2 kPa what is the pressure of the carbon dioxide gas alone?