Unit 1: January 26

College of Engineering and Computer Science
Mechanical Engineering Department
Mechanical Engineering 370
Thermodynamics
Fall 2010 Number: 14319 Instructor: Larry Caretto
Solutions to Unit One Exercise – Properties of Pure Substances
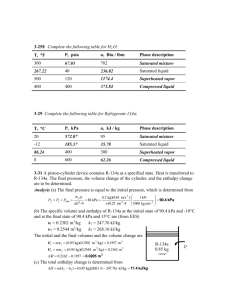
1. Find the specific volume of water at two points: (a) T = 600 o C and P = 50 kPa; (b) T = 360 o C and P = 20 MPa. At each point find the specific volume from the property tables for water and compare these results to those that you calculate from the ideal gas equation of state.
If either state is a compressed liquid, find the state from the compressed liquid tables and compare that result to the approximation that the specific volume of a compressed liquid is the same as that of the saturated liquid.
(a) `We can find the first state in the tables for superheated water. In Table A-6 on page 918 we find the specific volume at T = 600 o C and P = 50 kPa = 0.05 MPa is v = 8.0577 m 3 /kg .
To apply the ideal gas equation here, we use the value of the gas constant, R, for water from
Table A-1 on page 908: R = 0.4615 kJ/kg
∙
K. v
RT
P
0 .
4615 kg
K kJ
( 600
273 .
15 ) K
( 50
1 kJ kPa ) kPa
m
3
8 .
061 m
3 kg
In this case the ideal gas equation gives an error of 0.05% in the specific volume.
(b) In the first case, I just started looking in the superheat tables and found the desired state.
When I try this approach in the second case, I found the sub-table for a pressure of 20 MPa on page 921. There are no data for 350 o C and the first temperature shown in the table is
400 o C. However, in the same table, I can find that the saturation temperature at 20 MPa is
365.81
o C. (This is shown next to the pressure in the heading for each pressure table.) Since the given temperature of 360 o C is less than this we are in the liquid region. If we use the approximation that the specific volume at this temperature and pressure is approximately the same as the specific volume o the saturated liquid at the given temperature , we can look in table A-4 on page 9157 to find v f
(360 o C) = 0.001895 m 3 /kg.
However, we have compressed liquid tables for water. From Table A-7 on page 922 we can find that v =
0.0018248 m3/kg at 20 MPa and 360 o C. (In this case our approximation for a compressed liquid that v(T,P)
v f
(T) leads to an error of 3.8%.)
In this case, the ideal gas equation gives v
RT
P
0 .
4615 kJ kg
K
( 360
273 .
15 ) K
( 20 kPa )
1000 kJ
MPa
m
3
0 .
01461 m
3 kg
Jacaranda (Engineering) 3519
E-mail: lcaretto@csun.edu
Mail Code
8348
Phone: 818.677.6448
Fax: 818.677.7062
Unit one exercise solutions ME 370, L. S. Caretto, Fall 2010 Page 2
Since this is a liquid, we expect that the ideal gas equation will be far off and we are not disappointed. The ideal gas equation gives us an error of 701%! Notice that we can always make calculations with the ideal gas equation of state, but they may be useless.
2. Each state below is identified by a specific volume and a temperature or pressure. For each state find the temperature if the pressure is given or find the pressure if the temperature is given. If the state is in the mixed region, find the quality.
(a) Water, T = 600 o C, v = 0.2208 m
3
/kg
If we look in the tables for water at a temperature of 600 o C, we find the following data in Table A-
6 on page 919: at P
1
= 1.80 MPa, v
1
= 0.22200; at P
2
= 2 MPa, v
2
= 0.19962. The volume that we are looking for, v = 0.2208 m 3 /kg lies between these two values so that we have to interpolate to find the pressure. Using our general interpolation formula gives the following result.
2
P
P
1
MPa
1 .
80
P
2 v
2
MPa
P
1 v
1
( v
v
1
)
0 .
2208 m
3
1 .
80 MPa
0 .
19962 m
3 kg
0 .
22200 kg m
3 kg
0 .
22200 kg m
3
1 .
8077 MPa
So the interpolated pressure is P = 1.81 MPa .
(b) Refrigerant 134a, P = 500 kPa, v = 0.056205 m
3
/kg
If we look at the superheated vapor tables for R-134a on page 930 for the given pressure of 500 kPa
= 0.5 MPa, we find the given specific volume exactly, without interpolation, when T = 90 o C .
(c) Water, T = 200 o F, v = 20 ft
3
/lb m
At the given temperature of 200 o F, we find the specific volumes of saturated liquid and saturated vapor from the English unit tables on page 964. These are, respectively, v f
= 0.01663 ft 3 /lb m
and v g
= 33.613 ft 3 /lb m
, We see that our given volume is between these two so that we are in the mixed region. Since we are in the mixed region, the pressure is equal to the saturation pressure.
This gives P = P sat
(200 o F) = 11.538 psia . The quality is found as follows. x
v v
v f g
v f
33 .
20 lb ft
3 m
613 lb m
0 .
01663 lb m ft
3
0 .
01663 lb m ft
3 ft
3 x = 0.595
(d) Refrigerant 134a, P = 500 psia, v = 0.01201 ft
3
/lb m
Using the English-unit saturation table for R 134a (Table A-12E on page 977), we find that the specific volume of the saturated liquid for R 134a at a pressure of 500 psia is 0.01995 ft 3 /lb m
.
Since our given volume is less than this, we have a compressed liquid. There is no compressed liquid table for R 134a, so we will use the approximation that v(T,P)
v f
(T) for this point. This gives us v = 0.001201 ft 3 /lb m
= v f
(T). We have to find the temperature in the saturation table
(Table A-11E) for which the value of v f
matches our given volume. We see that our given value of v = 0.001201 ft 3 /lb m
is the table value at T = 10 0 F so we conclude that
T = 10 o F .
Unit one exercise solutions ME 370, L. S. Caretto, Fall 2010 Page 3
(e) Refrigerant 134a, P = 500 psia, v = 0.01204 ft
3
/lb m
Using the English-unit saturation table for R 134a (Table A-12E on page 977), we find that the specific volume of the saturated liquid for R 134a at a pressure of 500 psia is 0.01995 ft 3 /lb m
.
Since our given volume is less than this, we have a compressed liquid. There is no compressed liquid table for R 134a, so we will use the approximation that v(T,P)
v f
(T) for this point. This gives us v = 0.001204 ft 3 /lb m
= v f
(T). We have to find the temperature in the saturation table
(Table A-11E) for which the value of v f
matches our given volume. We see that our given value of v = 0.001204 ft 3 /lb m
lies between v f
= 0.001201 ft 3 /lb m
at T = 10 0 F and v f
= 0.001209 ft 3 /lb m
at T =
15 0 F. We find the temperature by Interpolating between these two points.
T
10 o
F
0 .
01209 lb
15 m o ft
F
3
10 o
F
0 .
01201 lb m ft
3
0 .
01204 lb m ft
3
0 .
01201 lb m ft
3
11 .
875 o
F
T = 12 o F .
3. Find the specific volume of R-134a at a pressure of 100 kPa and temperatures of (a) –25 o and (b) –30 o C.
C
If we guess that this state is superheated and look at Table A-13 on page 931 for superheated R-
134a, we see that that the saturation temperature (listed at the top of the table for each pressure) is -26.37
o C at the given pressure of 100 kPa. Since a temperature of – 25 o C is greater than the saturation temperature at the given pressure, we have a gas. But the first table entry is for a temperature of – 20 o C. Thus we have to interpolate between the saturated vapor data (T =
-26.54
o C, v = 0.19254 m 3 /kg) and the first temperature in the superheat table (T = – 20 o C, v =
0.19841 m 3 /kg). This gives v
0 .
19254 m
3 kg
0 .
19841 kg m
3
0 .
19254 kg
20 o
C
(
26 .
54 o
C ) m
3
25 o
C
(
26 .
54 o
C )
0 .
19393 m
3 kg
The second temperature of -30 o C is less than the saturation pressure at the given temperature which means that we have a compressed liquid. Here we can use the approximation that the specific volume of a compressed liquid is the same as the specific volume of the saturated liquid at the same temperature . This gives v(100 kPa, -30 o C) ≈ v f
(-30 o C) = 0.0007203 m 3 /kg from
Table A-11 on page 926.
4. The property tables used for quizzes have only one saturation table pressure as the intended look-up variable. Show that you can use this kind of table when the pressure is given by using Table A-5 to find the pressure and specific volume of water at a temperature of 220 o C and a quality, x = 0.25. Compare the result to the value of v =
0.022416 m 3 /kg from slide 25 of the August 26 lecture presentation.
Because we have a quality of 0.25 we know that we are in the mixed region. Since pressure and temperature are related in this region, we can use the temperature column in Table A-5 to find the given temperature of 220 o C. We find this on page 919, between 218.41
o C and 223.95
o C. We can then interpolate to find the saturation pressure (which is the system pressure for this mixed state) and the specific volumes of the saturated liquid and saturated vapor. This gives
P
2250 kPa
2500
223 kPa
2250
.
95 o
C
218 .
kPa
41 o
C
220 o
C
218 .
41 o
C
= 2321.8 kPa v f
0 .
001187 m
3 kg
0 .
001197 kg m
3
223 .
95 o
C
0 .
001187 kg
218 .
41 o
C m
3
220 o
C
218 .
41 o
C
0 .
001190 m
3 kg
Unit one exercise solutions ME 370, L. S. Caretto, Fall 2010 Page 4 v g
0 .
088717 m
3 kg
0 .
079952 m
3 kg
223 .
95 o
C
0 .
088717 kg
218 .
41 o
C m
3
220 o
C
218 .
41 o
C
0 .
085811 m
3 kg
All the interpolations above have the following factor in common:
220 o
C
223 .
95 o
C
218 .
41 o
C
218 .
41 o
C using a calculator we need only compute this factor one time.
; when
We can now find the specific volume from the usual equation involving the quality. v
1
x
v f
xv g
( 1
0 .
25 )
0 .
001190 kg m
3
( 0 .
25 )
0 .
085811 m
3 kg
=
0 .
022345 m
3 kg
The results obtained in the class example, using table A-4 with temperature as a look-up variable were P = 2319.6 kPa and v = 0.022416 m 3 /kg.
The following is an advanced topic which will not be covered further in this course. It does show how a non-linear interpolation formula, based on a physical equation, can give a more accurate interpolation result.
An alternative interpolation for saturation pressure is based on the Clausius-Clapeyron equation d(ln P)/d(1/T) = –h fg
/R; the temperatures in this equation must be absolute temperatures. If we assume that h fg
/R is approximately constant over the narrow interpolation interval we can use the following interpolation formula for the pressure. ln
ln P
ln P
1
P
2250 kPa
ln P
2
ln P
1
1
1
T
2
T
1 ln
2500
2250
1
T
kPa kPa
T
1
1
497 .
1
10 K
1
491 .
56 K
P
2250
ln
P
P
1
1
493 .
15 K ln
T
1
2
P
P
2
1
1
T
1
1
T
1
491 .
56 kPa
e
0 .
030481
= 2319.6 kPa
1
T
1
K
0 .
030481
This interpolation matches the five significant figures found in the saturation temperature table on page 917.
5. A story in the August 24, 2004 edition of the LA Times had the following statement:
“Science geek alert: The 7.62 round has a muzzle velocity of 2,750 feet per second and imparts to the 150-grain projectile a kinetic energy of about 2,511 footpounds.” Check this statement. Remember the conversion factor that 1 lb f
= 32.174 lb m
∙ft/s 2 . There are
7000 grains in a pound.
From the equation for kinetic energy, KE = mV 2 /2 we have the following calculation using the appropriate unit conversion factors.
KE
mV
2
2
1
2
150
7000 grains grains lb m
2750 s ft
2 lb f
s
2
32 .
174 lb m
ft
2518 ft
lb f
Which is “about 2,511 foot-pounds.”